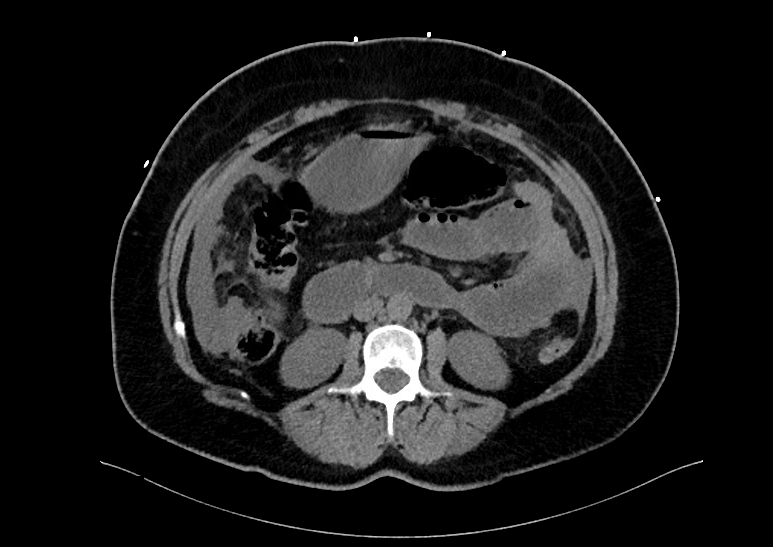
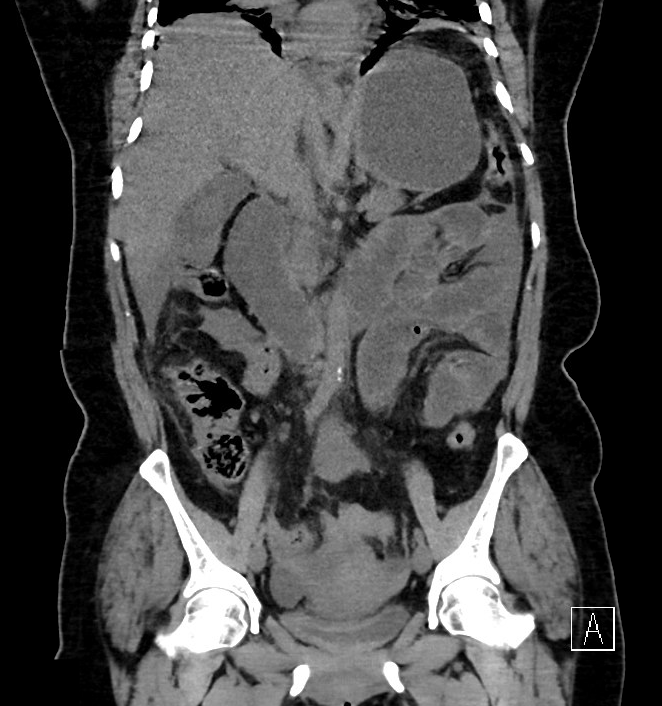
Case Presentation: A 55-year old female with no reported past medical or surgical history presented for evaluation of sharp epigastric pain radiating into her lower abdomen. The pain began two days prior upon awakening with associated bloating. She also reported nausea worsened by food but denied vomiting. Her last bowel movement was one day prior but she was still passing gas. She further denied fever, chills, history of kidney/liver problems, vaginal discharge. She underwent menopause one year prior.On admission, she was afebrile, blood pressure 98/67mmHg (mean arterial pressure 76), pulse 123bpm, saturating 98% on 4L nasal cannula. Physical exam showed tachycardia, clear lungs, positive bowel sounds, and no rebound tenderness. Blood count with differential showed white blood cells 6300 with significant bandemia of 41%, hemoglobin 11.4, platelets 466. Labwork was remarkable for bicarbonate 20, blood urea nitrogen 36, serum creatinine 3.41. Lactic acid was elevated at 4.0. Noncontrast CT abdomen/pelvis showed biliary sludge but no cholecystitis, small pelvic ascites, and early small bowel obstruction. She was started on empiric vancomycin, ceftriaxone, and metronidazole, as well as sepsis protocol fluid boluses with lactated Ringers and maintenance at 125cc/hour. She was then found to be COVID-19 positive via polymerase chain reaction. Unfortunately, she developed acute respiratory distress syndrome necessitating emergent intubation and ICU transfer. Antibiotics were broadened to Vancomycin, Meropenem, and Metronidazole. Given her unremarkable imaging, sudden decompensation, and no clear source of infection, an exploratory laparotomy was performed which revealed purulence to her peritoneal cavity. Culture of this fluid was sent to Mayo Clinic for evaluation and was positive for Fusobacterium necrophorum. She improved with 14-days of aggressive IV antibiotics, vasopressors, and mechanical ventilation, with subsequent discharge to inpatient rehabilitation.
Discussion: Fusobacterium necrophorum is an obligate anaerobic gram-negative bacillus often found in the gastrointestinal tract. To our knowledge, there have only been 3 other reported cases of primary peritonitis caused by this bacterium. Generally, primary peritonitis is associated with Streptococcus pneumoniae, Streptococcus pyogenes, or Neisseria meningitidis. However, F. necrophorum is the most common oropharyngeal anaerobe to cause sepsis and bacteremia, a condition known as Lemiere’s syndrome. This syndrome is described as oropharyngeal infection, extension into the lateral pharyngeal space, and septic thrombophlebitis of the internal jugular vein causing distant site abscess formation. Our patient’s cause of peritonitis remains unclear, as there were no obvious bowel perforations or oral lesions, therefore she did not meet the criteria for Lemiere’s syndrome. Possible explanations are related to her concomitant COVID-19 infection causing gastrointestinal distress with silent aspirationor microperforation not evident on the noncontrast CT abdomen/pelvis. However, her exploratory laparotomies failed to yield evidence of obvious perforation.
Conclusions: While primary peritonitis from F. necrophorum is uncommon, appropriate antibiotic coverage is essential in its management. It should be considered in patients with septic shock from primary peritonitis with negative routine cultures. Clinicians should choose anaerobic coverage and a β-lactam β-lactamase-resistant antibiotic.