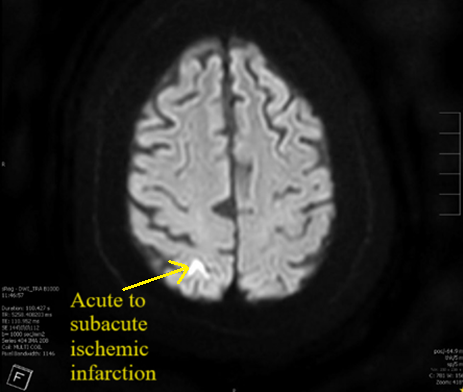
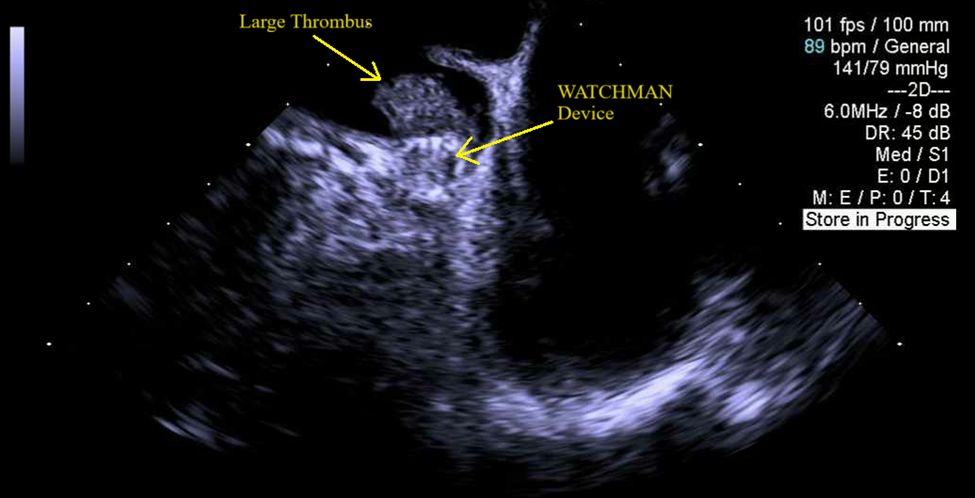
Case Presentation: A 76-year-old male with PMH of atrial fibrillation s/p WATCHMAN device three years prior to presentation, CAD, and COPD presented to the emergency department with altered mental status, generalized weakness, and dysarthria for one day. He denied having any headache, nausea, vomiting, trauma, numbness, tingling, or palpitations. On physical exam, his vitals were stable, he had dysarthria with no slurred speech, and he had no other focal neurological deficit. He was alert and oriented to person and place, but not time. The patient would have intermittent episodes of confusion during the interview process where he would occasionally forget his train of thought. The patient was admitted to the hospital to rule out stroke. His complete blood count, comprehensive metabolic panel, thyroid function tests, B12, folate, and ammonia levels were within normal limits. A CT brain without contrast revealed no acute findings. An MRI brain without contrast revealed small areas of acute to subacute ischemic infarcts in the right parietal occipital junction, left occipital lobe, and left frontal deep white matter (Figure 1). A subsequent transesophageal echocardiogram (TEE) demonstrated a large thrombus on the WATCHMAN device with an ejection fraction of 45% (Figure 2). The patient was started on heparin drip at the hospital and was later transitioned to apixaban. The WATCHMAN device was not removed, and the plan was to continue to medically manage his strokes and thrombus. The patient’s mental status and dysarthria improved throughout his hospital course.
Discussion: Left atrial appendage closure with a WATCHMAN device is essential in preventing thromboembolism in the left atrial appendage in patients with non-valvular atrial fibrillation and with contraindications for long term anticoagulation. Despite its intended purpose in preventing thromboembolism, the incidence of WATCHMAN device thrombus formation ranges from 3.7% to 6.6%. Thrombus formation typically occurs within the first year of implantation; however it can present years following placement. Device embolization is rare and occurs in roughly 0.24% of cases. Our patient was found to have multiple acute and sub-acute ischemic infarctions with WATCHMAN device thrombosis. Risk factors for device-associated thrombus formation include permanent atrial fibrillation, vascular disease, stroke, and low ejection fraction. Thrombus presence is typically demonstrated via TEE. Thrombi are managed with anticoagulation; however, there are no standardized guidelines regarding the choice and duration of anticoagulation for the management of WATCHMAN device thrombus. Traditionally, WATCHMAN device thrombus has been managed with warfarin. There have been some studies that have shown promising results with the use of direct oral anticoagulant. No specific guidelines are available in terms of removing WATCHMAN devices after thrombus formation has occurred.
Conclusions: A WATCHMAN carries the risk of device-related thrombus formation and physicians should consider thrombus development in patients presenting with embolic strokes.