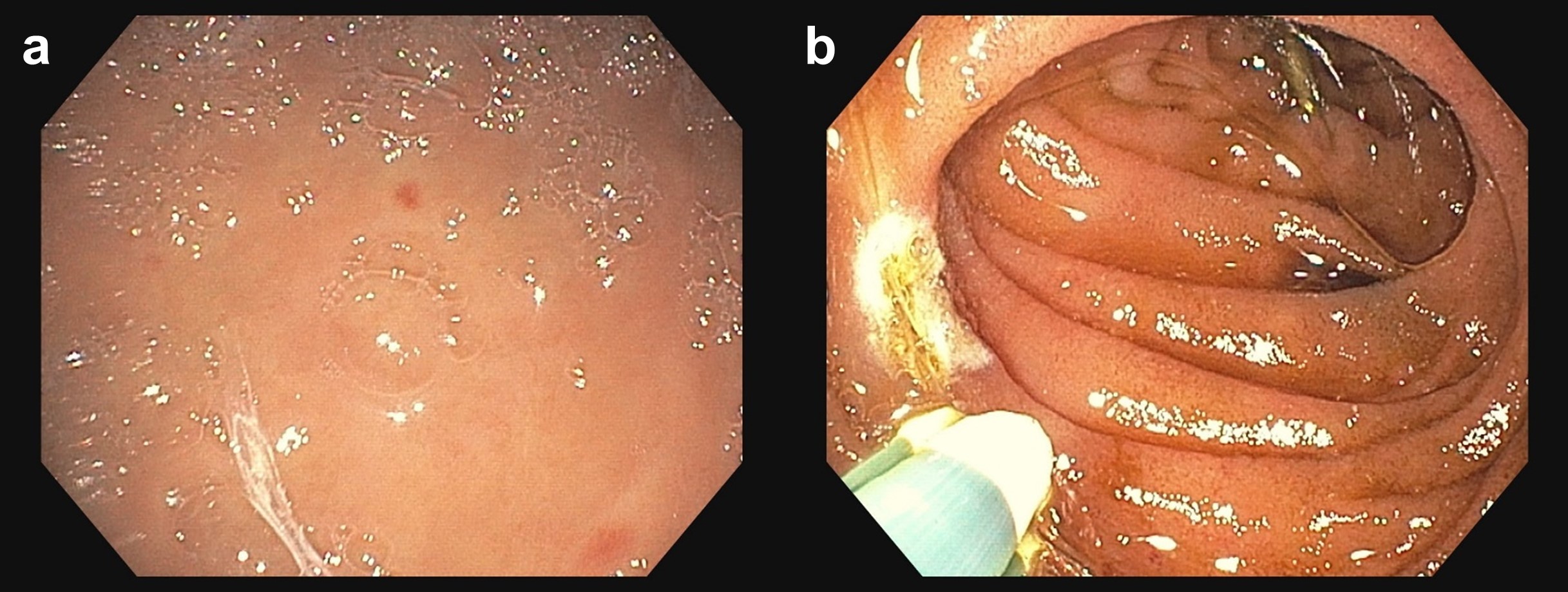
Case Presentation: A 75-year-old male with a history of gastrointestinal bleeding (GIB) due to angiodysplasia, aortic stenosis (AS) status post TAVR, moderate-severe mitral regurgitation (MR), CKD, GERD, and atrial fibrillation not on anticoagulation presented due to lightheadedness for 1 day when standing up, and fatigue for a few weeks. He also reported dark brown stools but no melena. He was admitted 4 months prior for similar complaints at which time endoscopy revealed bleeding angiodysplasia in the duodenum (2nd portion) and 2 large non-bleeding cecal angiodysplasia. He denied syncope, chest pain, dyspnea, vertigo, hematemesis, and hematochezia. He reported no NSAID use except aspirin 81mg, iron tablets, bismuth, international travel, significant alcohol consumption, and liver disease.He was vitally stable without jaundice but appeared fatigued. Cardiac examination revealed a systolic/holosystolic murmur at the left sternal border. Labs revealed a hemoglobin of 4.2 (9.3 two months prior) with a low-normal MCV (81.7). BUN/Cr ratio was 31. Liver chemistries and INR were overall unremarkable. LDH was elevated (238), haptoglobin was normal and peripheral smear revealed no schistocytes. Workup was suggestive of iron deficiency anemia. The patient’s presentation was thought to be due to an occult recurrent upper or small bowel GIB prompting RBC transfusions, IV PPI, and endoscopy. EGD revealed 2 non-bleeding gastric angiodysplasia and 4 bleeding angiodysplasia in the duodenum (2nd and 3rd portions) all treated endoscopically (Figure 1). Capsule endoscopy was suboptimal given retained melanic contents throughout the small bowel. Given the combination of recurrent occult GIB due to angiodysplasia, known prior valvular disease, and CKD, there was high clinical suspicion for Acquired von Willebrand syndrome (AVWS). The activity:antigen ratio for von Willebrand factor (vWF) was < 0.7 suggesting a structural or functional disorder. On multimer analysis, high molecular weight (HMW) vWF multimers were absent/decreased with no increased lower molecular weight vWF multimers suggestive of AVWS. On outpatient follow-up his hemoglobin remained stable and TEE showed moderate-severe MR where the aortic valve prosthesis transvalvular gradient was 14mmHg. Cardiology attributed the AVWS to the moderate-severe MR. They recommended observation with future evaluation for transcatheter mitral valve replacement.
Discussion: Gastrointestinal bleeding is a common cause of morbidity, mortality and hospital admissions in the elderly population. While the most common cause of upper GIB in the elderly is peptic ulcer disease, Heyde’s syndrome (HS) is a rare etiology that is difficult to diagnose resulting in recurrent bleeding and readmissions. HS is characterized by the unique relationship between severe AS and GIB from angiodysplasia. Specifically, the shear stress from valvular disease results in AVWS due to the destruction of HMW vWF multimers. In our patient, AS was unlikely to be the etiology given that TAVR is effective at preventing rebleeding. However, other causes for shear stress have been reported including LVADs, HOCM and we report a rare case due to moderate-severe MR. For such rare cases, mitral valve surgery or replacement can result in improvement of the AVWS.
Conclusions: Here we present a rare case of upper GIB secondary to HS. HS should be in the differential for recurrent GIB from angiodysplasia in the elderly, especially in the setting of known severe MR.