Background:
Neutropenic Fever (NF) is a common complication for patients on myelosuppressive chemotherapy. Despite guidelines by the Infectious Diseases Society of America (IDSA), however, there is variability in physician compliance. There are limited prior studies assessing compliance in this realm and those studies do not fully assess drivers of poor compliance. Poor compliance can result in increased morbidity and mortality, including increased risk of acquiring antibiotic resistant organisms and Clostridium difficile infection (CDI). The aim of this study was to gauge IDSA guideline compliance for treatment of NF at our institution, drivers of non-compliance, and associated outcomes.
Methods:
The study was conducted at a large tertiary care hospital, in which the majority of oncology patients are cared for by hospitalists. We conducted a retrospective chart review of NF admissions from 1/1/13-6/30/13. We identified 74 adult patients with Absolute Neutrophil Count (ANC) < 1000 and temperature > 100.4°F within 48 hours of admission to a cancer unit or ICU; 65 were managed for NF and included in analysis. We assessed guideline compliance using a six point score derived from IDSA recommendations: 1) correct definition of fever; 2) correct definition of neutropenia; 3) blood cultures before antibiotics; 4) appropriate empiric antibiotics; 5) appropriate antibiotics at 48-72 hours; and 6) appropriate antibiotics at day 4-7. Each parameter scored 1 if compliant. We defined a score of 6 as fully compliant, 4-5 partially compliant, and 0-3 non-compliant. Associated outcomes reviewed included: ICU transfer, inpatient mortality, adverse antibiotic reaction, and development of CDI or Vancomycin Resistant Enterococci (VRE) within 90 days of treatment for NF.
Results:
As shown in the Table, treatment was partially or fully non-compliant for the majority of NF patients and adverse outcomes were relatively common.
|
Compliance |
|
41.5% Fully Compliant (6) |
|
40.0% Partially Compliant (4-5) |
|
18.5% Non-compliant (0-3) |
|
Outcomes |
|
6.2% ICU transfer |
|
4.6% inpatient mortality |
|
4.6% adverse antibiotic reaction |
|
9.2% Clostridium difficile, 90 days |
|
7.7% new VRE, 90 days (60% infection; 40% colonization) |
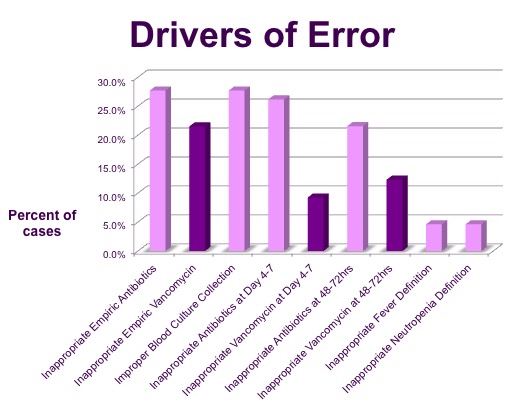
Regarding drivers of noncompliance, overuse of antimicrobials was common. Overall, 27.7%, 21.5%, and 26.2% of patients received improper antibiotics at admission, 48-72 hours, and day 4-7 respectively. At day 4-7 there was no indication for antibiotics at all in 41.2% of patients who were receiving inappropriate antibiotics.
Vancomycin use without indication was a common driver of improper antibiotics at all time points and was the reason for improper antibiotics in 77.8%, 57.1%, and 35.3% of cases at these time points respectively.
Altogether, patients received 126 doses of vancomycin with no indication (mean 5.5 doses per patient).
At day 4-7 there was no indication for antibiotics at all in 41.2% of patients who were receiving inappropriate antibiotics.
Conclusions:
Despite IDSA’s NF guidelines, compliance is poor and appears to be mainly driven by vancomycin overuse. In light of the relatively high rate of adverse drug reactions, new Clostridium difficile and VRE cases, further intervention is needed to improve compliance with established NF guidelines.