Background:
Anemia is a common medical problem in hospitalized patients. For several decades parenteral iron was available only as high molecular iron dextran preparations which were associated with frequent serious adverse reactions including anaphylaxis. Despite the introduction of safe intravenous (IV) iron formulations in the last two decades many clinicians still perceive them as dangerous therapy and underutilize it. Many studies in the outpatient setting have demonstrated IV iron replacement to be an effective and safe therapy for select patients with iron deficiency and blood loss anemia. However the use of IV iron in the inpatient setting was evaluated only in a few studies, limited to perioperative and post‐partum patients.
Methods:
This retrospective study was done in a single community hospital of 200 beds. A clinical pharmacist identified all inpatient orders for parenteral iron from July 1 2011 until June 30 2012. The electronic medical records of the identified patients were reviewed by the authors. When follow up hemoglobin levels were available the closest to 14 days after treatment was used.
Results:
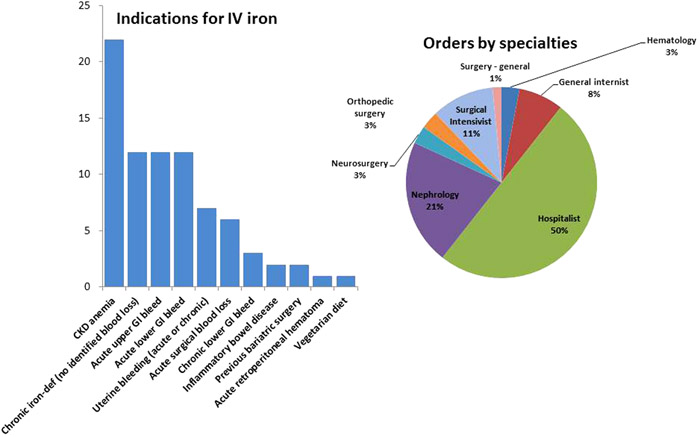
Sixty six patients were identified to have been prescribed IV iron and there were no reported adverse events. The mean total elemental iron infused for each patient was 429mg, hemoglobin level at treatment initiation was 8.7g/dL, MCV was 83.8fL, ferritin was 172.9ng/mL (median of 97.5), iron saturation was 15.4% and TIBC was 288.4ug/dL. Follow up hemoblobin level (mean of 13.26 days after treatment initiation) was 9.94g/dL and the increase from the baseline was statistically significant (P<0.0001). Twenty five patients (37.8%) also received blood transfusion (mean of 3.12 units of packed blood cells) with a mean baseline hemoglobin level of 8.32g/dL and a follow up (after a mean of 12.4 days) hemoglobin of 9.62g/dL. Patients who were not transfused (41, 62.2%) had a baseline hemoglobin of 8.94g/dL and a follow up (after a mean of 13.6 days) hemoglobin of 10.1g/dL. Patients who received ferrous gluconate (Ferrlecit) (56, 84.8%) had a mean total dose infused of 366.6mg. Patients who received low molecular weight iron dextran (Infed)(10, 15.1%) had a mean total dose infused of 947.5mg. The most common anemia etiologies were chronic kidney disease (12,18.1%), acute lower (11, 16.6%)and acute upper (11,16.6%) gastrointestinal bleeding and menorrhagia (7, 10.6%). Four patients (6%) had inflammatory bowel disease and 2 (3%) had previous bariatric surgery. Hospitalists were the prescribers for 33 patients (50%). One hospitalist prescribed IV iron for 28 patients (42.4%), while 2 others prescribed it for 2 patients and 2 prescribed it for one patient. Other 7 hospitalists did not prescribe intravenous iron during this 12 month period. The other patients had intravenous iron prescribed by nephrologists (14 patients, 21%), surgical intensivists (7 patients, 11%), general internists (5 patients, 8%), hematologists, orthopedic surgeons and neurosurgeons (2 patients each, 3%) and general surgery (1 patient, 1%).
Conclusions:
Use of intravenous iron is safe in hospitalized patients with diverse acute medical problems. Large variability is observed in utilization of this therapy amongst inpatient providers and most clinicians underutilize it. Possibly due to its better side‐effect profile and no need for a test dose, ferrous gluconate is more frequently ordered than iron dextran at this single center. Prospective trials are needed to study the impact on outcomes and cost‐effectiveness.