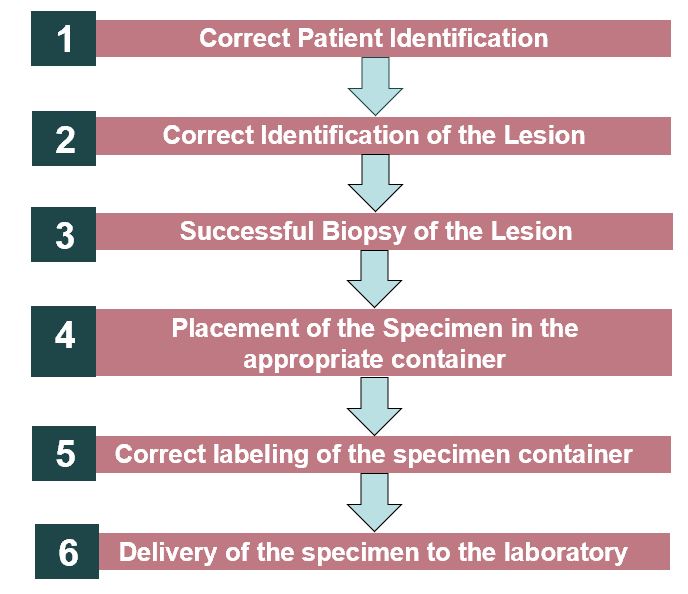
Background: Lost or improperly labeled specimens may be medically detrimental to patients and have legal implications for the medical professionals and healthcare facility involved. Endoscopy is an especially high-risk location for specimen errors given the large number of biopsies obtained and the high volume of patients served daily by the same team. A 61-year-old male presented for upper endoscopy and colonoscopy due to a 2-month history of nausea, vomiting, loose stools and abdominal pain. During the procedure, biopsies were obtained from several sites including the antrum of the stomach, duodenal bulb, and second portion of the duodenum. In this case, no tissue was present within the duodenal bulb specimen container. However, two tissue fragments of duodenal mucosa with no diagnostic abnormality (consistent with the duodenal bulb specimen) was found in the antrum of the stomach specimen container. Within the same month, a 66-year-old female presented for upper endoscopy evaluation due to dysphagia to solids and liquids. During the procedure, tissue biopsies of the proximal and distal esophagus were obtained to assess for eosinophilic esophagitis. The distal esophagus specimen cup was reported to have no tissue. The proximal esophagus specimen cup had three pieces of normal squamous mucosa.Specimen collection, labeling, and processing is highly complex involving multiple team members with significant variation between institutions and among departments within the same institution. Steps that have been identified as essential to successful specimen collection include: 1. correct patient identification, 2. correct identification of the lesion, 3. successful biopsy of the lesion, 4. placement of the specimen in the appropriate container, 5. correct labeling of the specimen container with demographic information/MRN, date, organ/tissue site, and lastly 6. delivery of the specimen to the laboratory. An error can occur at any step in this process.
Purpose: The objective of this quality improvement project was to decrease specimen errors in the endoscopy suite by standardizing the specimen collection process and building in multiple verification strategies rooted in the patient safety universal reliability skill S.T.A.R (Stop-Think-Act-Review) in order to reduce specimen errors.
Description: The verification strategies implemented include: 1) a two staff member verification with visualization of the tissue in each specimen cup, 2) a department stand-down held to emphasize continuous communication between staff managing the specimen cup and the endoscopist regarding adequacy of tissue sample obtained, 3) a sample read-back at the end of the procedure to address any labeling error discrepancies, and 4) removal of filled specimen cups between procedures to prevent tissue contamination between cases.
Conclusions: We anticipate tracking specimen error rate over the next year to determine effectiveness of this standardized process across the Sanford Fargo GI department and the effectiveness of each verification strategy for the collection process. The hope is to continue to refine this process as each error is analyzed. We hope that through this process, we continue to refine our specimen collection process ensuring safer outcomes for our patients. The strategies enacted have potential utility in specimen collection in other settings (ie. pap smears outpatient) and may result in the development of a new standard of care.