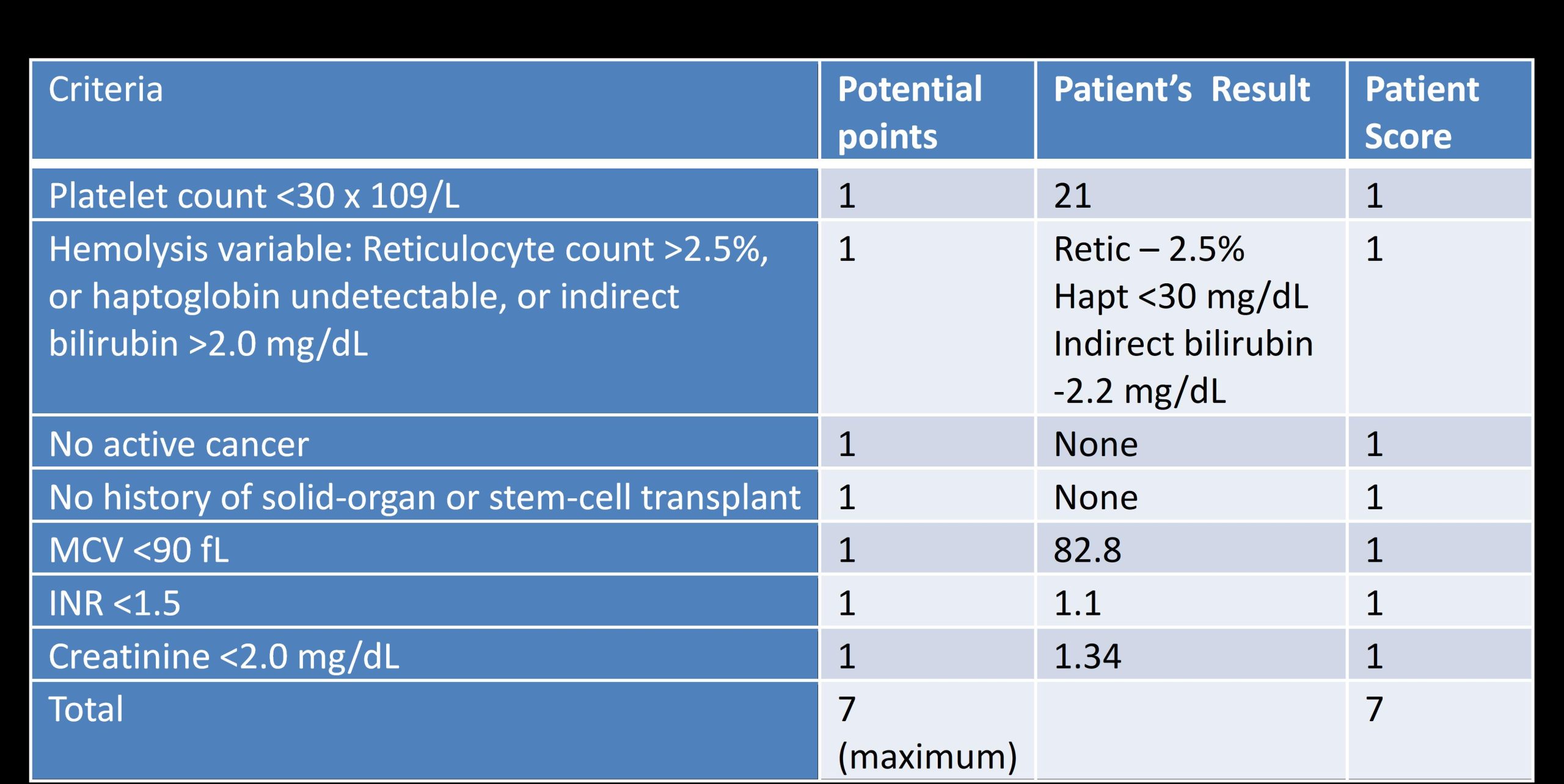
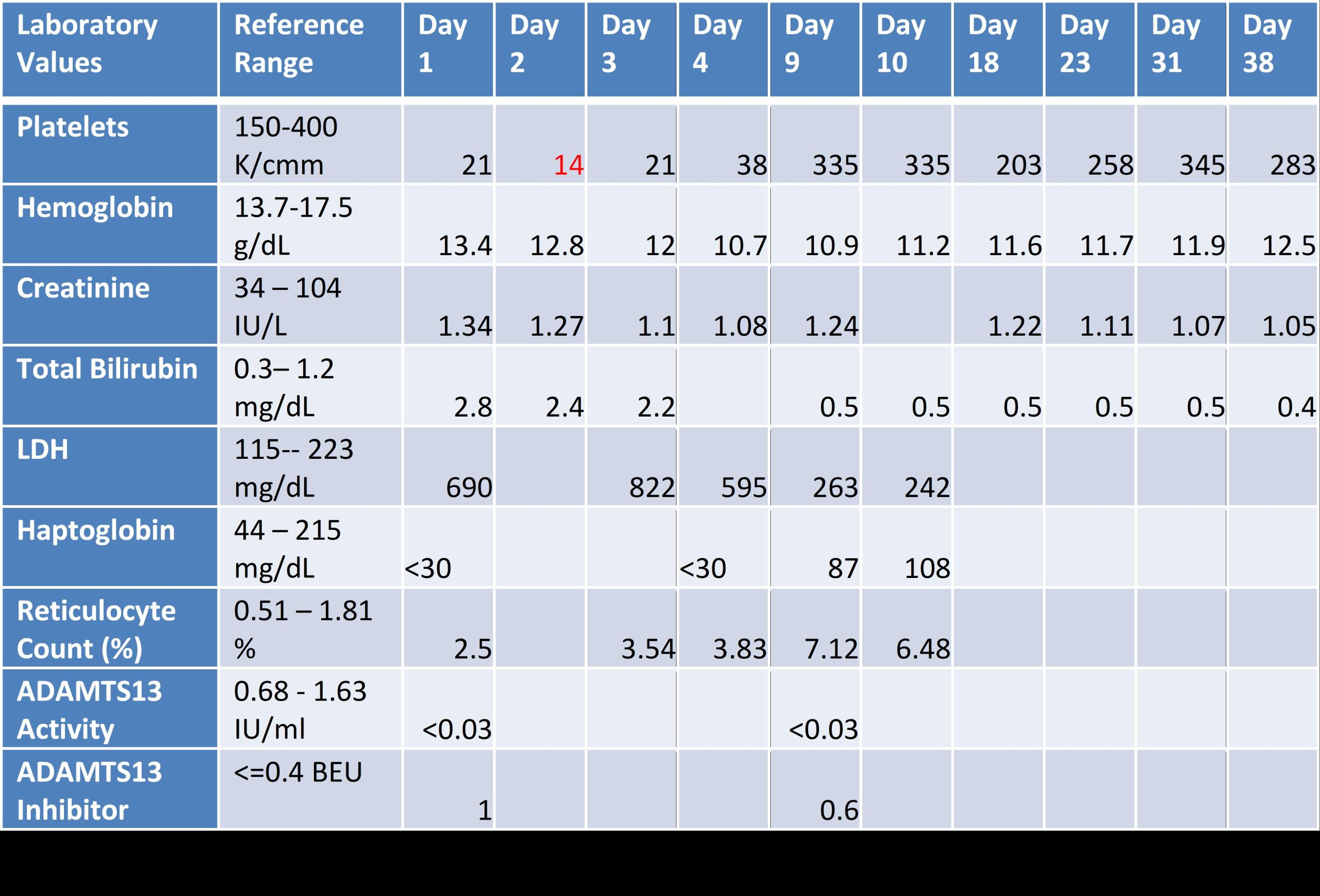
Case Presentation: A 54-year-old man presented with resolving epigastric pain. Initial labs revealed platelets at 21K/uL. He had no symptoms, signs of bleeding, history of easy bruising or hematologic disease. Further labs showed LDH 690U/L, haptoglobin < 30mg/dL, and bilirubin 2.8mg/dL (indirect 2.2), consistent with intravascular hemolysis. Hgb was 13.4g/dL with MCV 83fL and reticulocyte count 2.5% (mild response to hemolysis). Peripheral blood smear revealed large platelets with elliptocytes and few schistocytes. CMV IgM and Hep B core and surface antibody were positive. We sent ADAMTS13 activity and inhibitor levels with low suspicion for Thrombotic Thrombocytopenic Purpura (TTP).Due to lack of symptoms and few schistocytes on blood smear with thrombocytopenia, we initiated dexamethasone 40mg for 4 days under the working diagnosis of immune thrombocytopenic purpura (ITP). The next days, platelet nadir was 14 while Hgb fell to 10.7 with increased schistocytes on peripheral blood smear. PLASMIC Score used to predict ADAMTS13 deficiency was 7/7 (using Day 1 labs) indicating high risk of severe deficiency (< 10%) in TTP. With lack of symptoms, we dismissed indication for plasma exchange.On day 4, patient left against medical advice due to lack of symptoms with no definitive diagnosis. On day 7, ADAMTS13 level resulted as < 3% with inhibitor of 1.0, confirming immune-mediated TTP. Patient returned to the hospital on day 9. At this point, platelets rose to 355, yet Hgb was 10.9 with ongoing hemolysis and elevated reticulocyte count of 7.1%. Given normal platelets, we opted for Rituximab weekly for 4 weeks with Entecavir for HepB reactivation prophylaxis. After 4 weeks, platelets and hemolysis markers normalized while Hgb improved to 12.5.
Discussion: Acquired TTP is a life-threatening medical emergency requiring plasma exchange. Severe ADAMTS13 deficiency (activity ≤10%) confirms the diagnosis, however, confirmatory bioassays are not always readily available. The wide spectrum of symptoms presents a challenge for diagnosis and rapid treatment. A diagnostic (PLASMIC) score is a tool that has recently been validated with a high sensitivity to predict the likelihood of severe ADAMTS13 deficiency according to specific criteria . Our case shows the utility of the PLASMIC score in predicting TTP and prompting treatment in ambiguous cases when ADAMTS13 bioassays are not available. Our patient scored 7/7 on the PLASMIC score, an indication for rapid plasma exchange. However, it could be due to the asymptomatic nature that steroid use and rituximab were sufficient for treatment. In our patient’s asymptomatic phenotype of TTP, it is unknown whether CMV was a trigger, but it is unlikely as prior reports of CMV-induced TTP were only found in immunocompromised patients. The lack of symptoms made it especially challenging to convey the potential for lethal TTP-related complications. Fortunately, our patient was able to return to the hospital for further treatment with no consequences. While our patient recovered without it, initiation of plasma exchange in patients at high-risk for TTP remains critical as lifesaving therapy.
Conclusions: TTP is a rare life-threatening medical emergency. In cases of ambiguity and lack of readily available confirmatory bioassays, it is necessary to use the PLASMIC score to predict risk of ADAMTS13 deficiency. Accordingly, for patients with high risk of TTP, initiating plasma exchange prior to receiving ADAMTS13 levels must be considered to avoid severe TTP related complications.