Background: Guidelines recommend symptom triggered therapy for management of alcohol withdrawal. The most commonly use assessment scale is the Clinical Institute Withdrawal Assessment, Revised (CIWA-Ar). CIWA-Ar has been criticized however for relying on patient self-report, and alternative scales have been developed that incorporate vital signs to be more objective, including the Minnesota Detoxification Scale (MINDS) and the Severity of Ethanol Withdrawal Scale (SEWS). Few studies compare alcohol withdrawal outcomes based on the type of severity score used.
Methods: We conducted a retrospective cohort study of patients hospitalized with alcohol withdrawal on medical or surgical wards in 19 Veteran Health Administration (VHA) hospitals between October 1, 2018, and September 30, 2019. Clinical characteristics including demographics, comorbidities, risk factors for complicated withdrawal and factors related to the management of alcohol withdrawal were compared between patients managed using CIWA-Ar and severity scales that included vital signs. Logistic regression was used to examine factors associated with complicated withdrawal, while negative binomial regression was used for treatment duration. Factors significant on univariable analysis (P< 0.05) were included in final models.
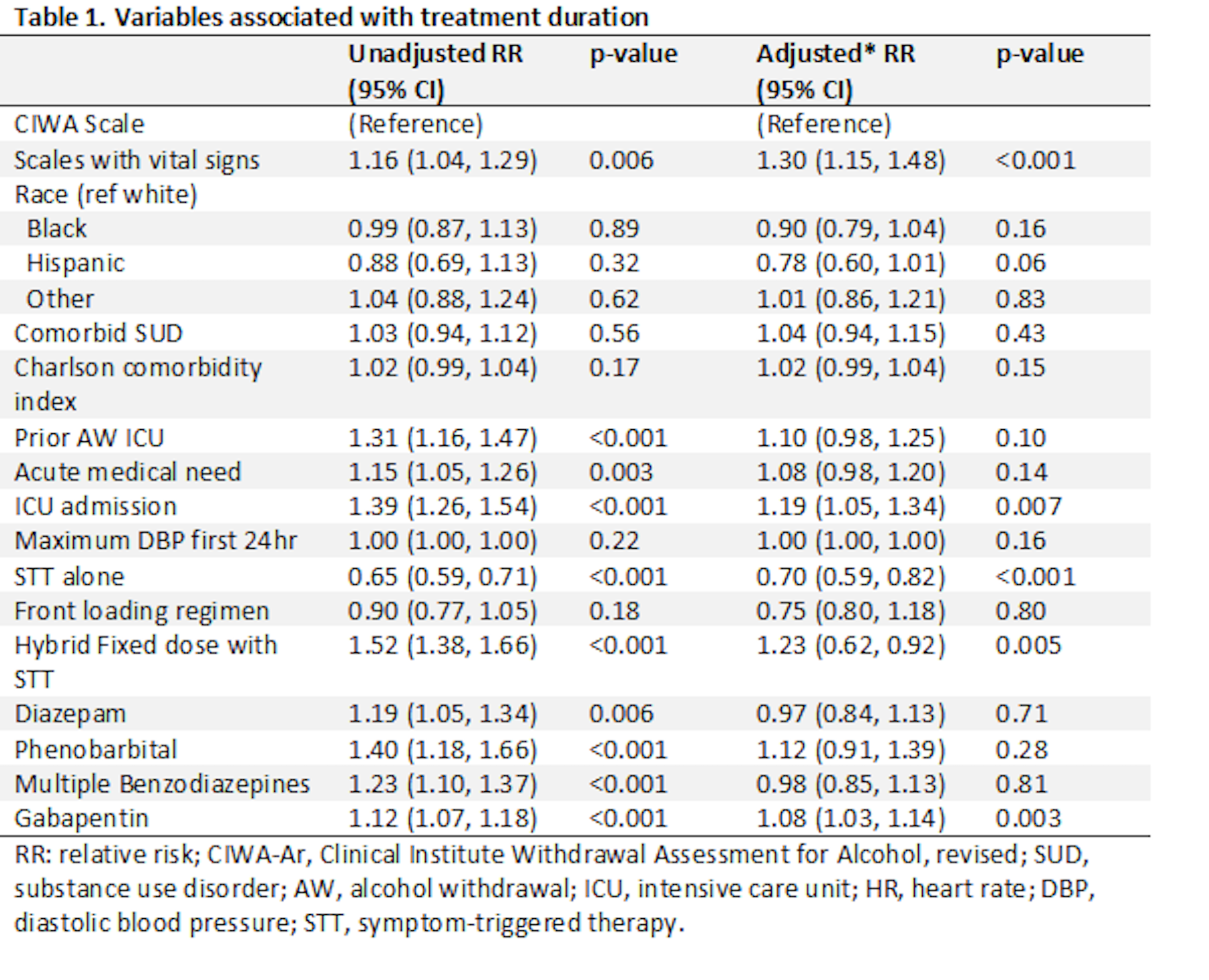
Results: In total, 461(78%) patients were managed using CIWA, and 127 (22%) patients were managed using symptom scales that included vital signs. The average duration of treatment was 3.4 days (SD, 2.0) for patients managed with CIWA compared to 3.9 days (SD, 2.0) for patients managed with scales incorporating vital signs (P= 0.007). On multivariable analysis, duration of treatment remained greater using scales with vital signs (RR 1.30, 95% CI, 1.15-1.48). There was no difference in rate of complicated withdrawal (OR 1.00, 95% CI, 0.41-2.46).
Conclusions: Our results indicate that alcohol withdrawal scales that incorporate vital signs were not superior to CIWA-Ar and were associated with increased treatment duration. Ideally, the choice of severity scale would be supported by randomized clinical trials.