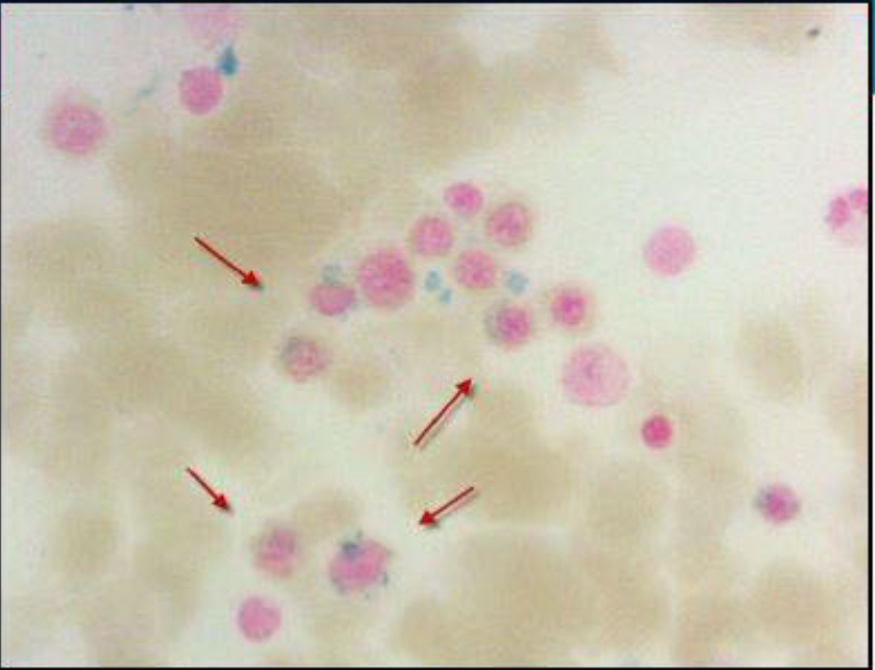
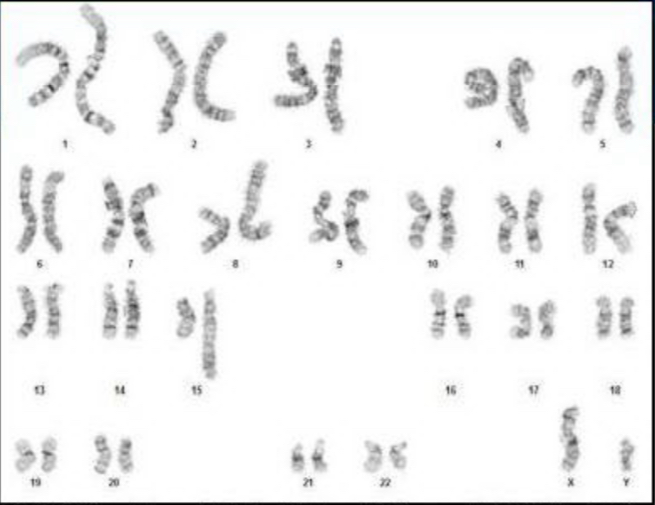
Case Presentation: A 19-year-old male in the United States Marine Corps (USMC) presented with severe fatigue and an inability to complete a 15-mile march. His initial hemoglobin (HgB) level was 2.5 g/dL, and his mean corpuscular volume (MCV) was elevated at 131.6 fL. After receiving 4 units of packed red blood cells, his HgB improved to above 7 g/dL. He had experienced fatigue for the past week, limiting his physical endurance. Anemia due to bleeding or nutritional deficiencies was ruled out. Notably, his military entry data showed an elevated glucose-6-phosphate dehydrogenase (G6PD) level of 643 Units/1×10^12 RBC, suggesting ongoing cellular turnover before his presentation.The patient had a small stature, midface hypoplasia, and café-au-lait spots on his skin. A bone marrow biopsy revealed hypercellular marrow with dysplasia and 3-4% blasts, indicating myelodysplastic syndrome (MDS). Additionally, 15-20% of red blood cell precursors displayed ringed sideroblasts. Molecular testing showed complex cytogenetics and a pathologic SF3B1 variant. Paroxysmal nocturnal hemoglobinuria was ruled out with negative flow cytometry.Chromosomal breakage testing using diepoxybutane (DEB) revealed an average of 6.22 DNA breaks per 50 cells, significantly above the normal range of 0.06/50 cells. An inherited Bone Marrow Failure Syndrome panel (iBMFS) detected three variants in the FANCA gene transcript, including one pathologic variant. The patient is currently under evaluation for allogeneic hematopoietic stem cell transplant.
Discussion: Fanconi anemia (FA) is a rare inherited bone marrow failure syndrome, with an incidence of approximately 1 in 300,000 live births. It is often diagnosed in the first decade of life, usually due to cytopenias or congenital anomalies. FA may also manifest later in life, with MDS or acute myeloid leukemia (AML) being common presentations.This case presents FA with atypical MDS, featuring concurrent high-risk complex cytogenetics and more favorable ringed sideroblasts, associated with an SF3B1 variant. Pathogenic mutations in the FANCA gene increase the risk of MDS and AML due to chromosomal instability. While MDS with complex cytogenetics is considered high risk for rapid transformation to AML, MDS with an SF3B1 mutation generally has a better prognosis.The SF3B1 mutation might have provided some protective effect for the patient, contributing to the delayed presentation of FA. Elevated G6PD activity during routine screening of servicemembers could be a valuable clue to an underlying hematologic disorder, prompting further investigation.
Conclusions: This case underscores a rare presentation of FA with an unusual MDS variant. The presence of high-risk complex cytogenetics and more favorable ringed sideroblasts linked to an SF3B1 variant complicates the clinical picture. Pathogenic mutations in the FANCA gene increase the risk of MDS and AML due to chromosomal instability.Notably, the presence of an SF3B1 mutation is associated with a better prognosis in MDS, potentially contributing to the delayed presentation of FA in this case. Moreover, elevated G6PD activity identified during routine screening could serve as a valuable clinical clue, indicating an underlying hematologic disorder that warrants further investigation. Recognizing such abnormalities may lead to earlier diagnosis and intervention, potentially improving outcomes for patients with rare hematologic conditions like FA.