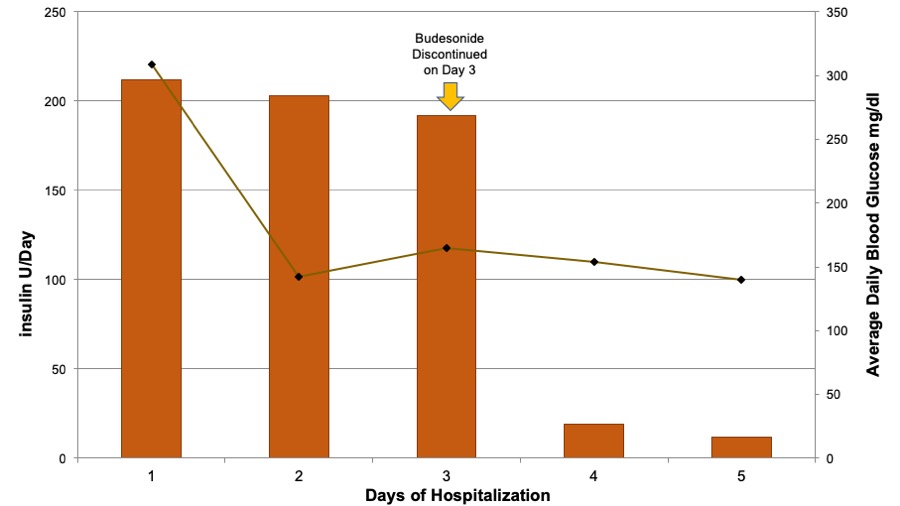
Case Presentation: A 62yo female with history of Combined Variable Immunodeficiency (CVID), Cirrhosis and Immune Mediated Colitis presented to the hospital with a 7-10 days history of confusion, ataxia, polyuria, and polydipsia. Her medications included Budesonide (9mg po once daily). Patient had no history of diabetes, and non-fasting blood glucose where in normal range 3 months preceding presentation. She had a Transjugular Intrahepatic Portosystemic Shunt (TIPS) performed 3 weeks prior to presentation for refractory ascites associated with cirrhosis. Work up revealed ammonia of 88 (at her baseline), positive influenza test, and Hyperosmolar Hyperglycemic State (HHS) with a blood sugar (BS) of 800mg/dl. Her HbA1c was 7.7%. Her initial insulin requirements were very high, at roughly 200U/day for the first 48 hours of her stay. After discontinuation of Budesonide on day 3 insulin requirements dramatically decreased to only 20U/day (Figure 1). On the 5th day she was discontinued from the drip and placed on a sliding scale, needing 12U/day. She was discharged on 500mg of Metformin twice a day.
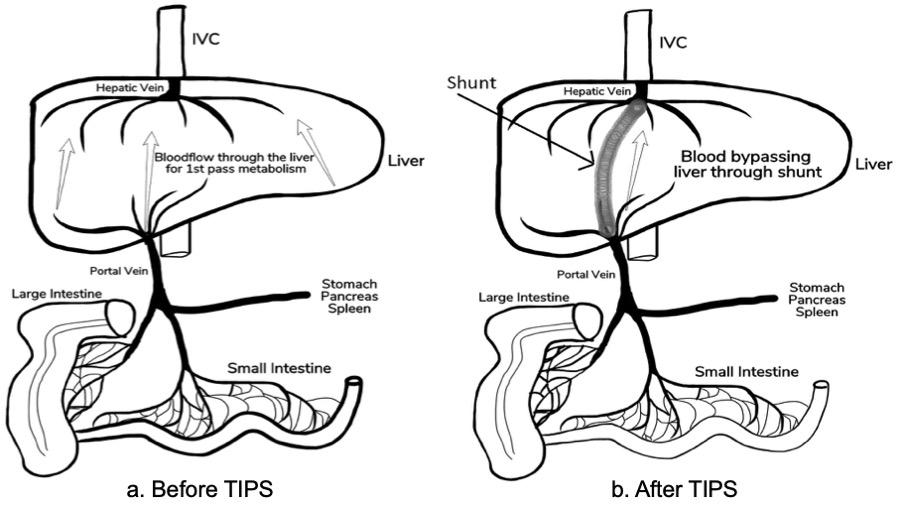
Discussion: It is a common misconception that budesonide is not absorbed when taken orally which is not true – it is absorbed, but then very quickly metabolized. It has an extremely high first pass metabolism resulting in minimal systemic glucorticoid effects(<1%). Development of HHS and dramatic insulin requirements within 3 weeks of TIPS leads us to postulate that the first pass metabolism of Budesonide was bypassed following TIPS leading to steroid induced diabetes (Figure 2). This is supported by the very abrupt and significant drop in insulin requirements upon its cessation.
Conclusions: The case beckons us to be mindful of major procedures that alter drug metabolism and make necessary adjustments to prevent patient complications.