Case Presentation: A 78-year-old female with a history of breast cancer and pancreatic cancer, with status post Whipple procedure and started with capecitabine one month prior to admission, severe malnutrition, started on a high-protein diet one month prior, presented with acute encephalopathy. Her symptoms started acutely as confusion, then progressed to somnolence. She was alert and oriented at baseline. This was her third admission for altered mental status within a month. She had no bowel movements for two days. Her vitals were stable, and she was responsive only to painful stimuli, with a GCS of 8. Her pupils were equal and reactive to light, and she had 2+ reflexes in all limbs. Further neurological exam could not be performed due to her mental status. Her exam was otherwise unremarkable. Her complete blood count and metabolic panel were unremarkable. She was found to have an ammonia level of 126 µmol/L. All cultures were negative. Thyroid function and blood gases were within normal limits. Urinary drug screen, serum acetaminophen, and salicylate levels were also negative. CT and MRI head showed only age-related changes. The EEG showed severe generalized slowing but no evidence of seizures. An abdominal MRI showed moderate fatty infiltration of the liver with no evidence of cirrhosis. Doppler studies showed no portal vein thrombosis.Lactulose enemas were started with improvement back to her baseline mental status. Rifaximin was later added given concerns regarding her ability to maintain 2-3 daily bowel movements with lactulose. She was discharged with both therapies with no recurrence of episodes.
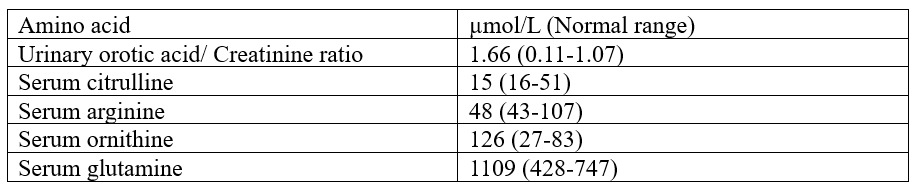
Discussion: Hyperammonemic encephalopathy after the administration of fluoropyrimidines has a reported incidence of 5.7% to 8.7%. Most reports show that encephalopathy follows the administration of high-dose 5-flurouracil. Seiichiro et al. found that 0.9% of cases followed its oral prodrug, capecitabine.(1) Oura, M., et al. found the reporting odds ratio of hyperammonemia to be 4.7 (95% CI 3.3–6.6) following capecitabine.(2)The exact mechanism of hyperammonemia in the setting of fluoropyrimidine therapy is unknown. Most cases report an aggravating factor, e.g., infection, dehydration, chronic constipation, or muscle loss.(3)Arriving at this diagnosis requires a thorough evaluation. Sources of increased ammonia, such as seizures, trauma, multiple myeloma, infections, and drugs, should be ruled out, as was done in this case. Sources of decreased ammonia elimination, such as portal vein thrombosis, adult-onset urea cycle disorders, carnitine deficiency, and medications like valproic acid, should also be considered.(4) In this case, the pattern of serum and urinary amino acids was not consistent with any of the cycle disorders.(5) Secondary carnitine deficiency was less likely as this patient had no hypoglycemic episodes during presentation.(6) The doppler of abdominal vessels also ruled out portal vein thrombosis.
Conclusions: It was concluded that the concurrent initiation of capecitabine and a high-protein diet in a patient who was severely malnourished with constipation provided the perfect recipe for hyperammonemia and eventually encephalopathy. This case allows a physician to recognise the thorough diagnostic work-up required to determine the etiology of hyperammonemia in a non-cirrhotic patient. It also forces one to recognise that the right co-morbidities can precipitate an otherwise rare complication of a drug.