Background:
Venous thromboembolism (VTE) is responsible for up to 600,000 hospitalizations per year in the United States. The appropriate use of evidence‐based VTE prophylaxis regimens, such as unfractionated heparin (UFH) or low‐molecular‐weight heparins (LMWHs), can reduce the clinical burden of VTE. We compared the clinical outcomes following appropriate prophylaxis with enoxaparin, the most frequently used LMWH, or UFH in a large, real‐world population of U.S. hospitalized medical and surgical patients at risk of VTE.
Methods:
Discharge records of patients aged ≥ 40 years at risk of VTE according to the 7th ACCP guidelines and who spent ≥6 days in the hospital were identified in the MarketScan® Hospital Drug database from Thomson Reuters (January 2004–March 2007). Patients were included in the study if they received appropriate (matching ACCP‐recommended prophylaxis type, dose, and duration) enoxaparin (at least 40 mg/day) or UFH (10,000 U/day) prophylaxis, with or without warfarin. Patients with contraindications to anticoagulation were excluded. Hospital‐acquired VTE events, pulmonary embolism (PE), deep‐vein thrombosis (DVT), and adverse events (including bleeding, with major bleeding defined as any intracranial bleeding or extracranial bleeding requiring transfusion of ≥2 units of red blood cells) were collected. Multivariate regression analysis adjusted for patient characteristics (age, sex, medical condition, type of surgery, number of clinical risk factors, and risk‐adjusted mortality index) and hospital characteristics (teaching status, licensed bed size, geographic region) was used to compare outcomes between the enoxaparin and UFH groups.
Results:
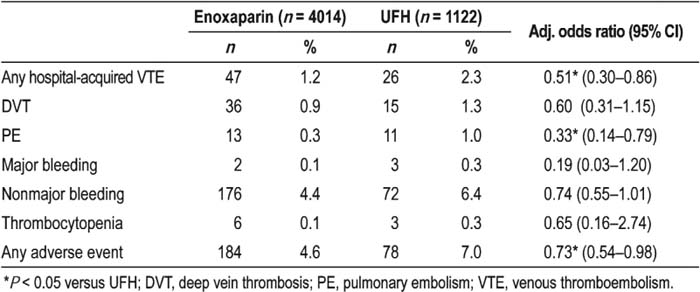
Of the patients reported in the 5136 discharge records included, 4014 (78%) received enoxaparin and 1122 (22%) received UFH. Following adjustment, enoxaparin was associated with a significantly reduced risk of hospital‐acquired DVT or PE compared with UFH (Table 1). The risk of any adverse event was significantly lower with enoxaparin than with UFH, although there was no significant difference in individual proportions of major or nonmajor bleeding (Table 1). Limitations of the study were that only readmis‐sions to the same hospital and within 30 days were included, and probably patients at higher risk of VTE were included because of the 6‐day minimum length of hospital stay.
Table 1. Proportion and Odds Ratios of Patients Experiencing Clinical Outcomes with Enoxaparin and Unfractionated Heparin (UFH)
Conclusions:
In patients at risk of VTE, enoxaparin appears to be associated with a lower risk of hospital‐acquired VTE events and adverse events than does VFH. Increased appropriate use of enoxaparin in ai‐risk patients may help to reduce the clinical burden of VTE.
Author Disclosure:
Alpesh Amin, sanofi‐aventis, speakers bureau, honoraria, research funding; Jay Lin, sanofi‐aventis, Employee; Greg Lenhart, sanofi‐aventis, Research Funding; Kathy Schulman, sanofi‐aventis, Research Funding.