Background:
Factor V Leiden (FVL) and prothrombin (PT20210A) gene mutations are common genetic polymorphisms that have been associated with first‐time venous thromboembolism (VTE). These mutations have been associated with recurrent thromboses in some but not all reported studies. We aimed to evaluate the clinical validity of these genetic markers for predicting recurrent VTE.
Methods:
As part of a larger project (AHRQ contract: HHSA 290‐2007‐10061‐1), we systematically reviewed English‐language studies that: (1) followed patients prospectively after an index VTE event; (2) tested patients at baseline for FVL, PT20210A, or both; (3) objectively diagnosed both the index thrombosis and any recurrence; and (4) reported both the number of subjects at risk and the number of recurrent events by mutation status. We searched MEDLINE, EMBASE, the Cochrane Library, and INAHL using a prospectively defined iterative search strategy designed to identify all relevant articles published between January 1993 and December 2008. Data were extracted independently by 2 investigators and adjudicated as necessary. We calculated pooled odds ratios using random‐effects models and tested for publication bias using funnel plots with trim‐and‐fill methods.
Results:
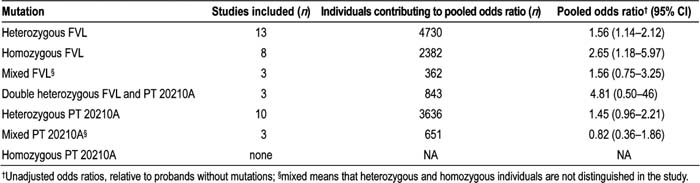
Of 12,815 potentially relevant citations, we identified 22 relevant articles, of which 13 had data appropriate for pooling. No significant publication bias was identified. Study quality was generally high. Heterogeneity among studies varied by the particular mutation examined. The FVL mutation was significantly associated with a modest risk of recurrent thrombosis (Table 1). The PT20210A mutation was not significantly associated with recurrence. In a subgroup analysis of studies that included only patients with idiopathic (unprovoked) thrombosis, the odds ratio for recurrence associated with heterozygous FVL was 0.85 [95% confidence interval (CI) 0.37–1.94]. However, this was not statistically different from the odds ratios in the studies that included both idiopathic and nonidio‐pathic thromboses: 1.88 (95% CI 1.42–2,47).
Conclusions:
We found a modest but significant association between the presence of FVL and the odds of recurrent VTE. We found no significant association between PT20210A and thrombosis recurrence. Given the modest odds ratios, studies are needed to explicitly evaluate testing individuals with thrombosis for these mutations to see if outcomes change if clinicians and patients know the testing results.
Author Disclosure:
D. Brotman, Canyon Pharmaceuticals, Advisory Board; sanofi‐aventis Pharmaceuticals, advisory board; Cubist Pharmaceuticals, Advisory Board; Quantia Communications, LLC, Hospitalist Leadership Panel; Bristol‐Myers Squibb/Sanofi Pharmaceuticals Partnership, advisory board; EMCREG International, advisory board; Otsuka America Pharmaceutical, Inc., Advisory Board; J. Segal, none; E. Bass, none; L. Wilson, none.