Background: In an effort to improve the design of future noninferiority studies, the endpoints for registrational trials of antimicrobials for the treatment of community-acquired bacterial pneumonia (CABP) were recently updated [1]. Lefamulin (LEF) is a first-in-class systemic pleuromutilin antibiotic newly approved for the treatment of adults with CABP [2]. The efficacy of LEF was demonstrated in 2 global phase 3 noninferiority clinical trials, LEAP 1 and LEAP 2, using the updated US Food and Drug Administration (FDA) endpoint [1,3,4]. Here, we report rates of concordance, positive predictive value (PPV), and negative predictive value (NPV) of the endpoint of early clinical response (ECR) with the endpoint of investigator assessment of clinical response (IACR) at test of cure (TOC) using data from the LEAP studies.
Methods: In LEAP 1, adults with CABP (Pneumonia Outcomes Research Team [PORT] risk class III–V) received LEF 150 mg IV every 12 hours (q12h) for 5–7 days or moxifloxacin (MOX) 400 mg IV every 24 hours (q24h) for 7 days, with optional IV-to-oral switch (600 mg LEF q12h or 400 mg MOX q24h) [3]. In LEAP 2, adults with CABP (PORT II–IV) received LEF 600 mg oral q12h for 5 days or MOX 400 mg oral q24h for 7 days [4]. The study protocol and amendments were approved by an independent ethics committee or institutional review board at each study site, and each patient (or legally authorized representative) provided written informed consent. Both studies assessed ECR at 96±24 hours after first study drug dose in the intent-to-treat (ITT; all randomized patients) population (US FDA primary endpoint) and IACR at TOC 5–10 days after last study drug dose in the modified ITT (mITT; received ≥1 study drug dose) and clinically evaluable (met predefined evaluability criteria) populations (European Medicines Agency coprimary endpoints). For ECR vs IACR at TOC, concordance (sum of the proportions of IACR success/ECR responders and IACR failure/ECR nonresponders) and test characteristics (sensitivity, specificity, PPV [proportion of IACR successes at TOC that were also ECR responders], and NPV [proportion of IACR failures at TOC that were also ECR nonresponders]) in the ITT population were determined.
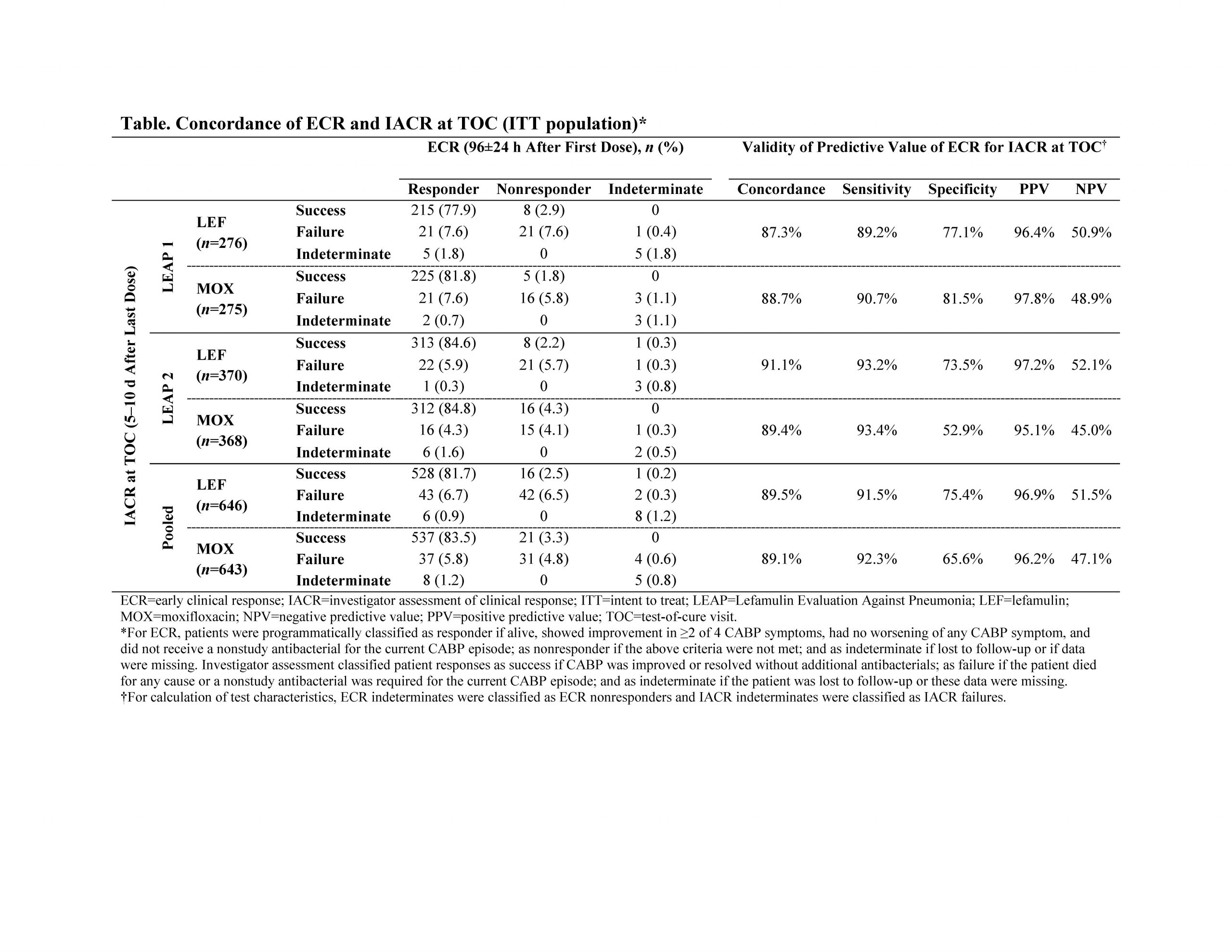
Results: The pooled analysis of LEAP 1 and LEAP 2 included 1289 patients (LEF, n=646; MOX, n=643). In both trials, LEF was noninferior to MOX based on ECR and IACR at TOC. In the pooled ITT population, high concordance between ECR and IACR at TOC was observed in the LEF (89.4%) and MOX (89.1%) treatment groups, with ECR (at 96±24 hours) displaying a sensitivity of ≥91% and a PPV of ≥96% for subsequent IACR success at TOC (Table). Concordance (87.3%–91.1%), sensitivity (≥89%), and PPV (≥95%) were similarly high within each individual study and treatment group. NPV ranged from 45.0%–52.1%, indicating that ECR nonresponse at 96±24 hours does not predict IACR failure at TOC.
Conclusions: Consistent with the literature [5], these analyses demonstrate that a favorable clinical response at 96±24 hours after the first dose of LEF among patients with CABP in the LEAP trials was a good predictor for clinical success 5–10 days after the last dose of LEF. This robust association of ECR with IACR success at TOC, combined with the ease of switching patients from IV to oral LEF [2], may facilitate improved transition of care from the inpatient/emergency department setting to the outpatient setting.