Background: The COVID-19 RT-PCR test is a real-time reverse transcription polymerase chain reaction (rRT-PCR) test for the qualitative detection of nucleic acid from SARS-CoV-2 virus (8). False negative RT-PCR tests pose a serious challenge as they can lead to diagnosis and treatment delays as well as increased risk of viral transmission in the community (1-10). We sought to understand the rates of false negative RT-PCR tests in our urban, academic safety-net hospital and characterize the patient population with negative initial tests for COVID-19 and subsequent positive testing.
Methods: Verified duplicate SARS-CoV-2 tests were reviewed from 3/28/2020-8/31/2020. Patients with initial negative tests followed by a positive test within 96 hours were included. Nasopharyngeal (NP), oropharyngeal (OP), and nasal swab (NS) collections were tested in-house using various molecular platforms or sent to reference laboratories. Patient charts were retrospectively reviewed for demographic and clinical information. Patients <18 years old or with initial testing done for routine asymptomatic pre-procedural testing were excluded. Descriptive statistics were computed for all demographic variables. χ² analyses, paired t-tests and Wilcoxon Rank Sum tests compared clinical and testing characteristics between study subjects’ initial and subsequent COVID test results. P-value < 0.05 was considered significant.
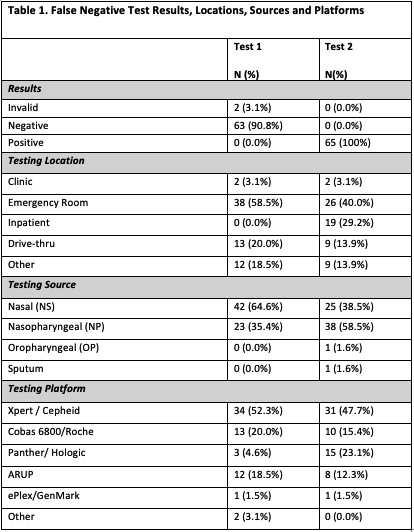
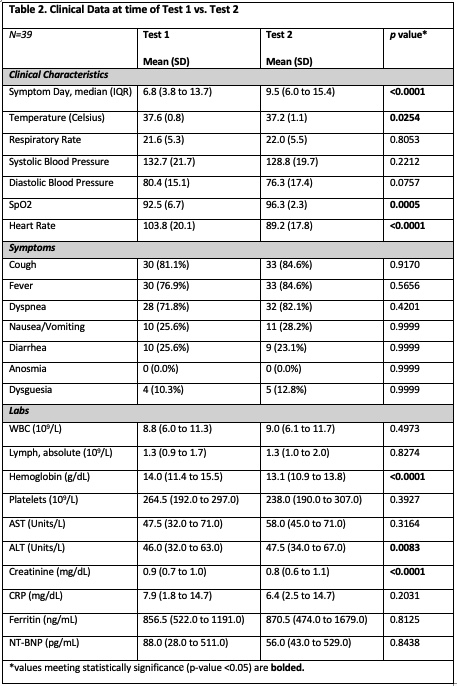
Results: A total of 16,196 duplicate SARS-CoV-2 PCR tests were reviewed over the 5-month study period. We identified 74 individuals with positive results within 96 hours of their initial negative test, and 65 (87.8%) met inclusion criteria. Clinical information was available for 39 (60.0%) of the 65 patients. Of the test 1 group, 64.6% (n=42) were NS while the remainder were NP. Of the test 2 group, 40% were NS and 57% were NP (Table 1). When the first test was a NS, 52.4% (n=22) were re-tested via NP method. Conversely, initial NP tests were followed by only 18.9% (n=7) NS. A median of 1.9 days separated tests 1 and 2, with 40.0% (n=26) being performed at >72 hours and 24.6% (n=16) performed <24 hours. Of the 39 patients evaluated for the clinical characteristics, 66.7% (n=26) were male and 71.8% (n=28) were Hispanic. Hospitalization was required for 84.6% (n=33). The mean symptom day at time of test 1 was 6.8 days vs. 9.5 days at time of test 2. The most common symptoms were cough, fever, and shortness of breath. While some vitals and labs reached statistical significance, no clinically significant differences were identified between test 1 and test 2 (Table 2). Empiric antibiotics were initiated in 48.7% of patients and continued in 18% of patients at 48 hours. Of admitted patients, 54.5% (n=19) were deemed patients under investigation despite a negative test.
Conclusions: Our study reflects the complex and rapidly evolving area of COVID-19 testing. The mean symptom day for positive results was 9.5, which is later than previously reported studies. Patients with false negative tests appear to present with similar symptoms to the majority of COVID-19 patients without clinical evolution at second testing. Despite prior research suggesting comparable results with NP and NS testing, providers appear to have greater confidence in NP swabs, sourcing NPs more frequently for second tests. Our results indicate a high prevalence and duration of empiric antibiotics for patients presenting with COVID-like symptoms but having false negative results, which has important implications for antimicrobial stewardship.