Background: Hospital at home (HaH) programs have shown efficacy as substitutes for high-cost, traditional hospitalization in patients who are primarily low-acuity and with a range of medical conditions, including community-acquired pneumonia (CAP) and urinary tract infection (UTI). However, wide-spread adoption is minimal as providers and patients are challenged to consider HaH at the time of hospital admission in a fast-paced, high-acuity environment, such as the Emergency Department (ED). In analogous scenarios, risk model-informed clinical decision support has helped drive implementation. Thus, we sought to create, validate, and test a risk prediction model using clinical data that is readily available at the time of admission decision making and hypothesized the proposed risk model can accurately identify low risk patients who may be appropriate for HaH services.
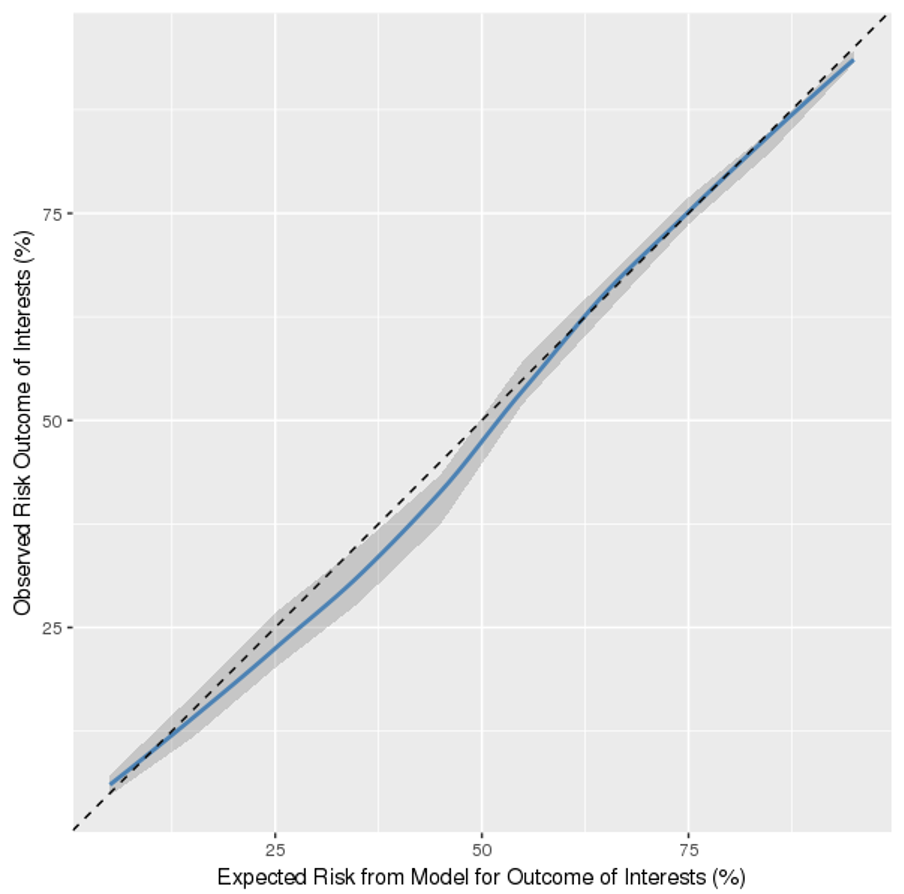
Methods: We identified patients hospitalized via the ED with suspected CAP or UTI to any of 12 affiliated acute care hospitals of the healthcare system in the Southeast of the U.S. between January 2014 and September 2017. We included adults (≥18 years) with suspected CAP (antibiotics, chest X-ray/CT ordered in ED) or UTI (antibiotics, urine culture, and urinalysis ordered in ED). We applied exclusion criteria similar to published HaH guidelines: 1) hospitalization in the last 30 days; 2) chemotherapy in the last 90 days; or 3) history of advanced HIV in the last one year. We captured electronic health record data on initial physiologic and laboratory values in the ED and sociodemographic factors, diagnoses, and prior healthcare utilization. The primary outcome was a composite of 1) all-cause 30-day Mortality; 2) Early critical intervention (i.e., receipt of mechanical or non-invasive ventilation, or vasopressors within first 72 hours); or 3) Length Of Stay > 72 hours (MELOS). We split the cohort into training (57109 admissions in 2014-2016) and testing (17783 admissions in 2017) datasets. In the training data, we constructed a multivariable logistic regression model using a backward elimination approach for variable selection (prespecified threshold of p>0.1). We performed k-fold cross validation (k=10) to assess internal model validity. To minimize false negative classifications in predicting patients at low risk of MELOS, we selected model performance thresholds to optimize negative predictive value (NPV) > 99%. In the testing data, we evaluated model discrimination using area under the receiver-operating characteristic curve (AUC) and NPV. We generated a calibration plot to examine differences in observed versus predicted event rates.
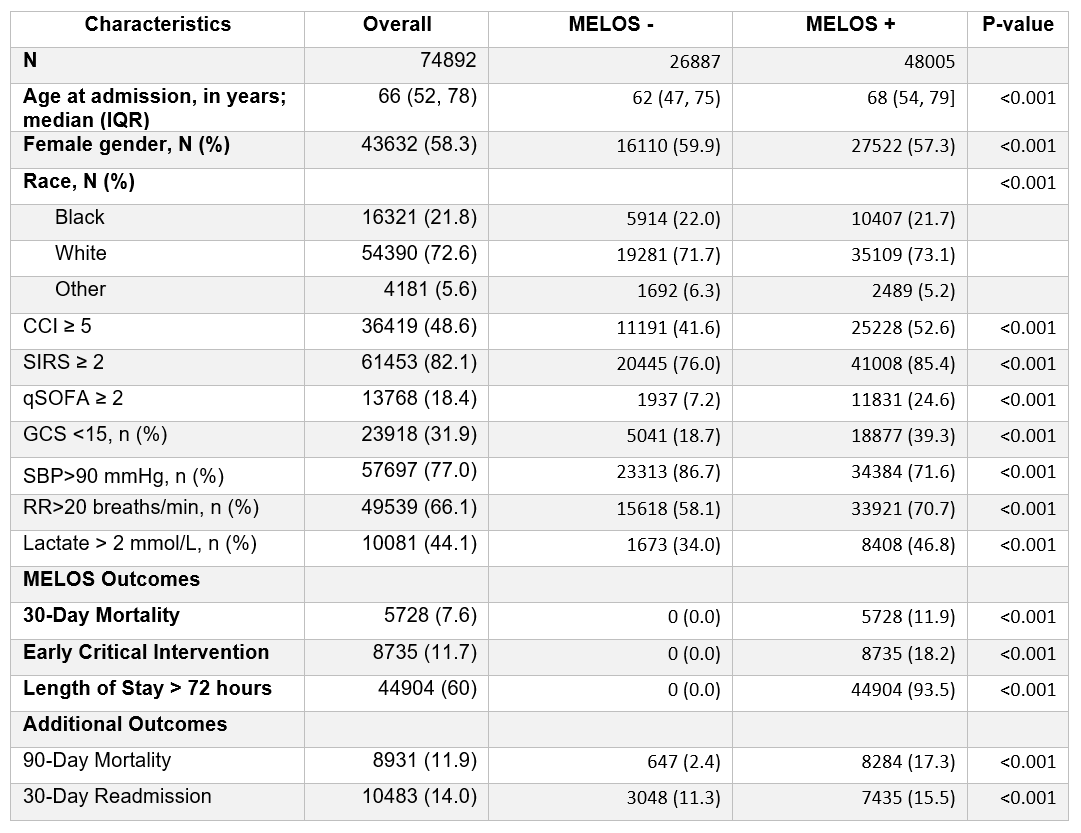
Results: Of 74,892 patients admitted with suspected CAP or UTI, 26,887 (36%) were MELOS free and considered the target population for HaH. MELOS free patients had fewer comorbidities and abnormalities in physiologic or laboratory values, compared to MELOS positive patients (p<0.001). There were 95 variables selected in the final risk model (from 175). In the testing dataset, AUC=0.84 (CI=0.83-0.85) and NPV =0.98, with the threshold setting to 0.02. The calibration plot (Figure 1) showed the model underpredicted MELOS when the predicted score was between 20% and 50%.
Conclusions: Our HaH risk model demonstrated the capability to identify patients at low risk of adverse outcomes, who may be suitable candidates for HaH. This model may inform selection of HaH eligible patients, thus improving implementation of HaH. As a next step, we will prospectively validate risk model performance.