Background: Closing the gap between evidence-supported antibiotic use and prescribing patterns among clinicians is a vital component of curbing excessive antibiotic use, a practice that fosters antimicrobial resistance and exposes patients to the side effects of antimicrobial agents. Providing medication prescribing information via scorecard has been shown to improve clinician adherence to quality metrics in a number of clinical settings. Our objective is to develop a set of metrics that can be extracted from the electronic medical record and which provides relevant and actionable feedback to hospital medicine physicians about their antibiotic prescribing practices.
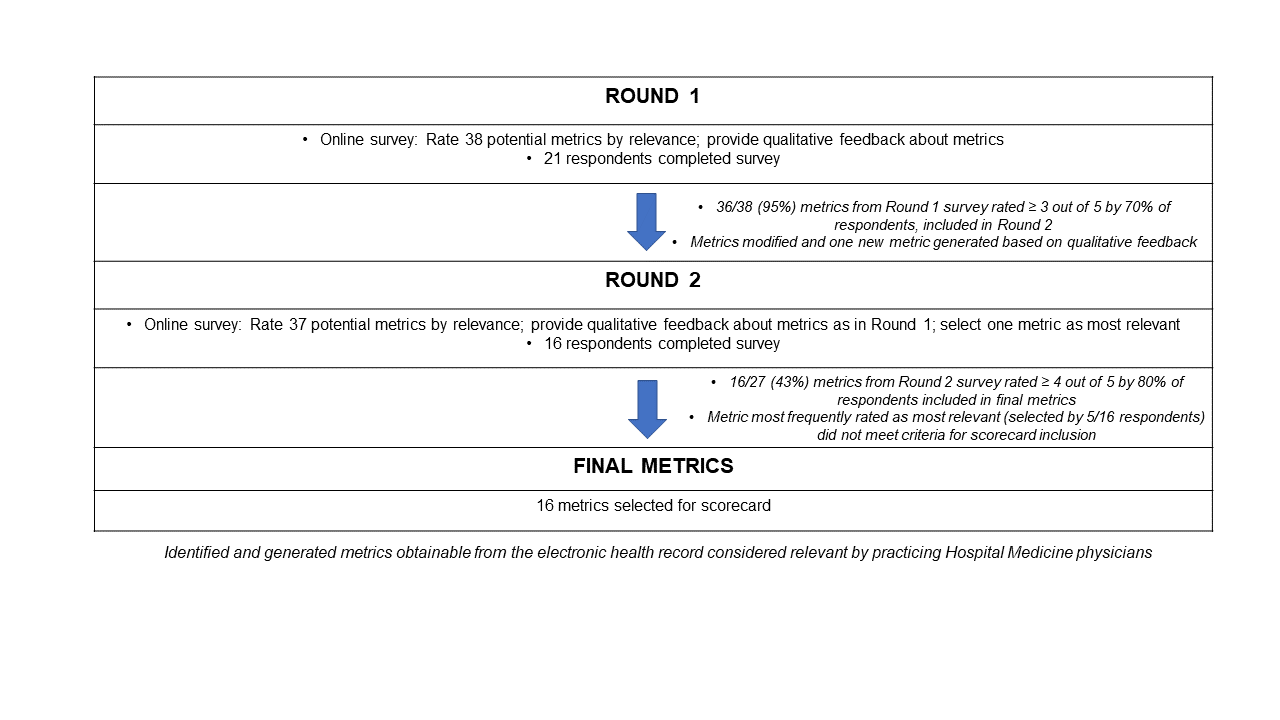
Methods: We used a modified Delphi consensus-building approach via two iterations of an electronic survey (Qualtrics, Provo, UT) disseminated to hospital medicine physicians via faculty listservs at 10 academic medical centers nationwide. We performed a literature review to identify actionable, feasible, and relevant quality indicators of antimicrobial prescribing patterns. We adapted these quality measures to ensure all metrics could be extracted from the medical record, compiling them into the first draft of the survey. Metrics on the first survey that met criteria for inclusion in the second survey were modified based on qualitative feedback, and respondents from the first survey were invited to take the second survey. Measures that met criteria for inclusion in the final scorecard were modified based on qualitative feedback from the second survey (Figure 1).
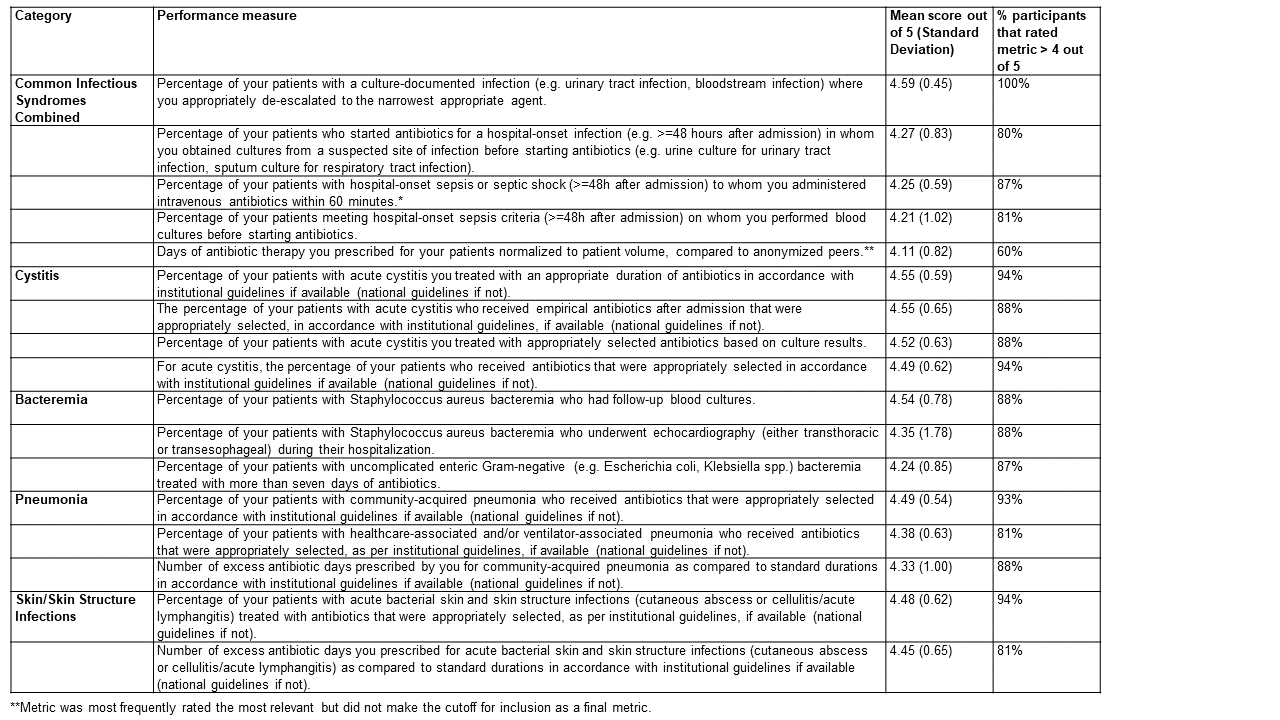
Results: Twenty-one participants from 10 institutions completed the first version of the survey and were affiliated with institutions located in the Pacific, Rocky Mountain, Southeast, Northeast, and Midwest United States. Metrics rated as most relevant included appropriate selection, de-escalation, and duration of antibiotics among patients with specific infectious syndromes, especially cystitis (Table 1). The metric most often selected as most relevant by respondents was the number of days of antibiotic therapy prescribed, corrected by patient volume. This metric did not meet criteria for inclusion in the final scorecard.
Conclusions: We used a consensus-building approach to identify 16 metrics that could provide individualized relevant and actionable feedback to hospital medicine physicians about their antibiotic prescribing practices. Metrics considered most relevant by hospitalists focused on appropriate de-escalation of antimicrobial therapy in patients with an infection, as well as appropriate treatment of acute cystitis. Next steps will involve prioritization and implementation of these metrics locally based on existing quality gaps at our institution; focus group discussions to explore impressions of clinicians who receive a scorecard; and analysis of antibiotic prescribing patterns before and after implementation of the scorecard.