Background: Proton pump inhibitors (PPI’s) are a commonly used class of medications, whose optimal use is important. Aim: To assess if proactive surveillance in inpatients can reduce PPI use for unapproved indications and their effect over 90 days post-discharge.
Methods: Inpatients were approached as part of a QI project at our tertiary-care center for evaluation of PPI use. Data on demographics, indication and the duration of the PPI use were reviewed. Two team members adjudicated appropriateness of PPI indication vis-à-vis the FDA guidelines. PPI changes included stopping, dose reduction or switching to H2 blockers after thorough counseling of the patients in communication with primary care physicians at discharge. Patients were followed for outcomes (PPI use patterns, readmissions, GI bleeding, pneumonia and death) at 30 and 90 days. The outcomes were compared between those with/without FDA-approved indications and those who continued the same PPI dose at discharge compared to those whose doses were modified/stopped.
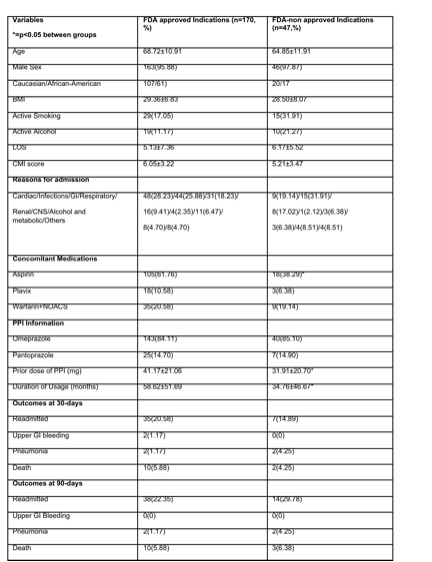
Results: Overall, 217 patients (age 67.88±11.22; 96% men; 63% Caucasian & 36% African American; BMI 29.18±7.11, LOS 5.4±7.0, Charlson Comorbidity Index (CMI) 5.87±3.29 who were on PPI’s at admission were included. The majority (84%) were on omeprazole & rest were on pantoprazole for 53.45±51.49 months with an average daily dose of 39.17±21.28 mg. The leading cause for PPI use was for GERD in 39 (64%) patients. FDA Indications: 47 (22%) patients had FDA unapproved indications for PPI use and hence, PPI was stopped in these while they were hospitalized and at discharge. We found170 patients on PPI’s with FDA approved indications. Out of these we were successfully able to decrease the dose in 31(14%), switch to H2 blockers in 15(7%) patients while no changes were made in122 (56%) patients.
Comparison between FDA Approved and Unapproved Indications (Table 1): There were no differences in demographics, reason for admission, LOS or CMI between groups. As expected, Aspirin use was highest in those on approved indications. Readmissions, pneumonia, GI bleeding were similar between groups at 30 and 90 days.
Comparison between those with changes in PPI dose/use vs those with intact PPI doses/frequency: Regardless of FDA approved/not, 93(42.85%) of total pts had either PPI withdrawal, reduction in dose or H2RA substitution compared to 124(57.14%) who did not. All outcomes were similar statistically; readmissions in PPI same vs changed were (24 vs 19), pneumonia in (0 vs 4), GI bleeding in (1 vs 1) and death in (1 vs 4) at 30 days. Readmissions in PPI same vs changed were (27 vs 25), pneumonia was seen in (2 vs 2), GI bleeding in (0vs 0) and death in (9 vs 5) at 90 days. The PPI dose had to be escalated back to the starting dose in 1(0.58) & 3(1.76%) patients respectively at 30 and 90 days.
Conclusions: Using an active surveillance program, judicious PPI use was ensured in most patients who did not qualify for long-term PPI use. This pattern was largely sustained even 90 days post-discharge and was associated with similar outcomes compared to patients in whom PPIs were continued