Background:
Glucagon‐like peptide‐1 (GLP‐1) receptors are present in human cardiac myocytes. Myocardial cells demonstrate insulin resistance in the setting of left ventricular dysfunction. Exenatide is a synthetic GLP‐1 mimetic molecule with insulinotropic and insulinomimetic properties. It has a favorable pharmacokinetic profile over GLP‐1. Insulin and GLP‐1 increase glucose utilization by cardiac myocytes and improve cardiac contractility. We hypothesized that a single subcutaneous dose of exenatide would improve the left ventricular ejection fraction (LVEF) of patients with stable CHF and an LVEF ≤ 40%.
Methods:
We investigated the short‐term efficacy and safety of a single dose of exenatide in patients with an LVEF ≤ 40%. A single 5‐μg subcutaneous dose of exenatide was given to 7 patients who were previously on standard heart failure medication for at least 6 weeks. These patients acted as their own controls. The primary end point was change in LVEF, and secondary end points were end‐systolic volume index (ESVI), end‐diastolic volume index (EDVI), peripheral blood sugar, and hemodynamic response (systolic blood pressure, diastolic blood pressure, heart rate, and mean arterial pressure). Base line LVEF assessment was done with a MUGA scan with standard radioactive isotope dose and technique, and a repeat MUGA scan was done 1 hour after the administration of 5 μg of subcutaneous exenatide. This study was HIPAA compliant. The hospital institutional review board approved conducting this pilot, nonrandomized single‐center study. Seven of 10 patients were able to complete the study. Data were analyzed using the paired t test and the independent t test and are presented as mean ± SEM. The P value was 2‐tailed, and a value < 0.05 was considered statistically significant. Statistical analysis was done using SPSS software.
Results:
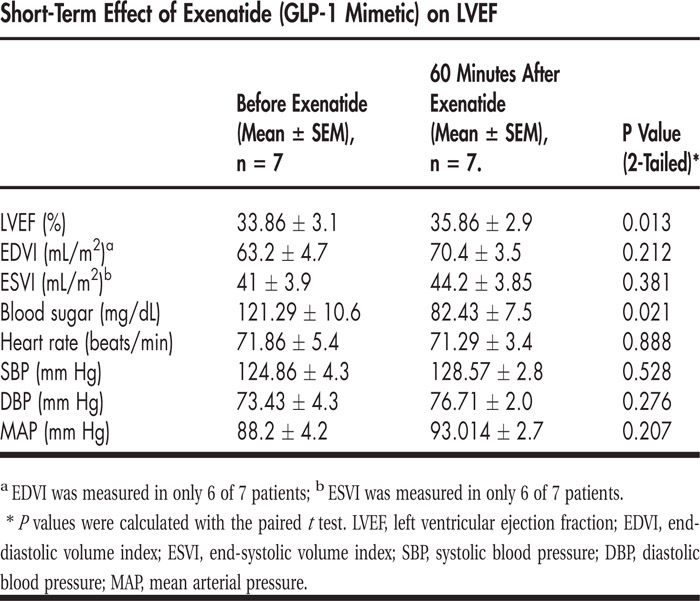
Single‐dose exenatide in immediate follow‐up increased the LVEF (from 33.86 ± 3.051 to 35.86 ± 2.915, P = 0.013) and decreased peripheral blood sugar (from 121.29 ± 10.58 to 82.43 ± 7.521, P = 0.021). There was no significant change in EDVI (from 63.2 ± 4.7 to 70.4 ± 3.5, P = 0.212), ESVI (from 41 ± 3.9 to 44.2 ± 3.85, P = 0.381), heart rate (from 71.86 ± 5.378 to 71.29 ± 3.414, P = 0.888), and mean arterial pressure (from 88.2 ± 4.182 to 93.014 ± 2.71, P = 0.207). One patient had nausea, and 1 patient experienced hypoglycemia. There were no adverse cardiovascular events. All 7 patients completed the study.
Conclusions:
There was significant improvement in LVEF 1 hour after administration of subcutaneous exenatide in patients with an LVEF ≤ 40% who were on standard heart failure medications for at least 6‐weeks. No larger prospective human clinical trial has been conducted so far to elucidate the long‐term effects of GLP‐1 or exenatide on the stable heart failure population. Exenatide has provided promising results in our study, and it can be studied prospectively in a larger population, which is technically feasible.
Disclosures:
W. Y. Banday ‐ none; B.G. Rueda ‐ none; A. Herle ‐ none; H. Lippes ‐ Amylin Pharmaeuticals; Eli Lilly Co; Novo Nordisk ‐ speakers bureau