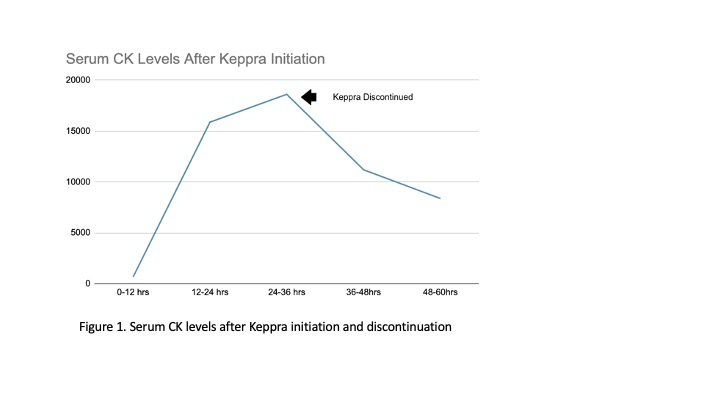
Case Presentation: A 41-year-old Caucasian female with a significant past medical history of Factor V Leiden deficiency presented to our institution for evaluation of new-onset seizures that began while driving. No head trauma or other bodily harm was sustained. The patient was non-ill appearing and hemodynamically stable. The physical exam was otherwise unremarkable. Laboratory evaluations were notable for CPK level of 671 and patient was started on IV fluids. Given new-onset seizures, the patient was loaded with Levetiracetam and continued a maintenance dose of 500mg bid. Serial CPK levels were obtained and after initially down trending, levels began to rise to a maximum level of 18616 by hospital day three. Given the continued rise in CK levels despite adequate hydration and urine output, Keppra was discontinued, as it was thought to be the offending agent. Serial CPK levels began to downtrend within the next 12 hours, supporting our suspicion.
Discussion: Levetiracetam, also known as, Keppra, is a second-generation antiepileptic medication that is commonly used to manage seizures in patients with generalized and partial seizures. Levetiracetam is generally considered safe, well-tolerated, and frequently used in patients in and out of the hospital. Rhabdomyolysis is a condition of muscle breakdown that can be present in patients with seizures.The mechanism of action of Levetiracetam involves binding to a protein in the brain called synaptic vesicle protein 2A (SV2A) leading to the release of several neurotransmitters, including GABA, glutamate, and dopamine, which can help to reduce seizures. Rhabdomyolysis is a serious condition in which skeletal muscle breaks down rapidly, leading to the release of myoglobin into the bloodstream and potentially causing kidney damage. One proposed mechanism of Levetiracetam-induced rhabdomyolysis involves the binding of SV2A, which has been shown to localize at motor nerve terminals, leading to increased muscle stress and rhabdomyolysis. To our knowledge, only a few cases of Levetiracetam-induced rhabdomyolysis have been reported. Most cases reported of Levetiracetam-induced rhabdomyolysis were observed within 12-36 hrs of initiation of the medication with a peak serum CK level 3-5 days after initiation and often resulted in symptoms such as muscle aches and dark urine. Our patient’s serum CK levels also peaked at day 3 however in our patient no symptoms of rhabdomyolysis were observed.
Conclusions: Levetiracetam is a frequently prescribed antiepileptic medication that is generally well tolerated. Although seizures on their own are known to cause muscle breakdown and elevations in CPK, a CPK that does not improve with appropriate fluid resuscitation should warrant further investigation into rare etiologies. Levetiracetam administration leading to rhabdomyolysis is a severe adverse effect for clinicians to be aware of and clinicians should maintain a high index of suspicion. Early recognition and discontinuation of Levetiracetam in the setting of rhabdomyolysis can avoid prolonged hospitalization, worsening kidney injury, and iatrogenic fluid overload.