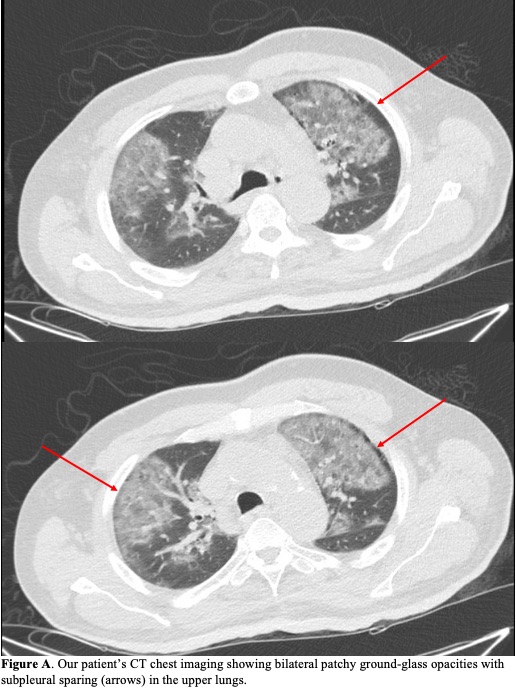
Case Presentation: A 63-year-old man with well-controlled human immunodeficiency virus infection, diabetes and peripheral artery disease presented with one day of hypoxemia and altered mental status (AMS). He was recently admitted for a right femoral-posterior tibial artery bypass angioplasty with excisional debridement of his foot. His course was complicated by an acute kidney injury (AKI), with creatinine (Cr) at 2.09 mg/dL on discharge (previous baseline 1-1.4 mg/dL). Tissue culture grew Morganella morganii and vancomycin-resistant Enterococcus faecium. He was discharged on a 4-week course of daptomycin and ciprofloxacin. Upon hospital presentation 7 days later, he was hypoxemic requiring nasal cannula with worsened AKI (Cr 6.65 mg/dL, BUN 69 mg/dL). CT chest showed bilateral upper-lung predominant patchy ground-glass opacities with subpleural sparing (Figure A). He was given intravenous fluids and antibiotics were adjusted to linezolid and ceftriaxone. Pneumonia work-up was negative for bacteria on sputum culture, viruses on respiratory pathogen panel, and serum Fungitell, aspergillus galactomannan, and cryptococcal antigen. While his AMS resolved with improving renal function, his hypoxemia persisted despite appropriate coverage before and during admission for infectious etiology, including aspiration pneumonitis. Thus he was given a short prednisone course for probable daptomycin-induced eosinophilic pneumonia (DIEP), after which his respiratory status returned to baseline. At the time of this report, weeks after discharge, he has finished his antibiotic course and remains at his nursing home.
Discussion: DIEP is a rare antibiotic adverse effect, often developing within a month of initiation and resolving after withdrawal and, sometimes, corticosteroid administration. Current Food and Drug Administration diagnostic criteria for DIEP requires all 6 of the following factors to be present: daptomycin exposure, dyspnea with increased O2 requirement, fever, new radiographic lung infiltrates, bronchoalveolar lavage (BAL) with >25% eosinophils, and clinical improvement following daptomycin cessation. Our case only met 4 criteria, which is similar to most prior reports as BAL is not regularly performed due to clinical risk and fever is inconsistent on presentation. Less restrictive algorithms prioritizing characteristic CT findings (bilateral interlobular septal thickening, alveolar consolidations, ground glass opacities, subpleural sparing, upper lobe and peripheral predominance) and peripheral eosinophilia have been proposed, though acknowledge that even lack of peripheral eosinophilia would not exclude diagnosis. Risk factors underlying DIEP include male sex, older age (>60 years), diabetes, obesity, and renal dysfunction—all factors present in our patient. Given that his infectious work-up was unremarkable and his respiratory status did not improve until corticosteroid administration, it was thought more likely that his hypoxemia was from DIEP. His worsened AKI compounded daptomycin toxicity risk and likely instigated his second hospital presentation, highlighting the importance of monitoring renal function.
Conclusions: We describe a case of probable DIEP that responded to corticosteroid use despite failing to meet current DIEP diagnostic criteria, in line with previous reports. This supports the growing need for a new diagnostic algorithm and increased awareness of DIEP as an etiology in patients with daptomycin exposure and negative infectious work-up.