Background: Transfusion of packed red blood cells in the United States has more than doubled from 1997 to 2011; however, it is thought that greater than 50% of transfusions may be unnecessary. Numerous clinical trials have demonstrated that restrictive transfusion strategies are noninferior or superior to liberal strategies across a variety of clinical scenarios; as a result, restrictive strategies are now recommended by most professional societies. At our institution, we are working to improve RBC utilization and compliance to national transfusions guidelines. Prior to intervention in Q3 2017, transfusion was directed primarily by provider clinical assessment with little-to-no clinical decision support. Our goal is to improve utilization of RBCs by increasing provider compliance to clinical guidelines. We expected reduction of unindicated RBC transfusions in our facility by at least 25% as well as an overall reduction in blood use of at least 10% within 12 months of initial intervention.
Methods: This quality improvement initiative was completed over 15 months (Q3 2017-Q3 2018), with baseline data collected prior. During this time, a blood utilization committee was established, comprised of representatives from medicine, surgery, nursing, blood bank, quality and safety, and pharmacy.
The plan-do-study-act (PDSA) methodology was utilized over two phases. In the first phase, the committee generated decision support tools that were implemented to our electronic ordering system in Q3 2017; it asked that an indication be entered for each transfusion from a pre-populated list of evidence-based indications. Following a run-in period, all transfusions over a 30-day period were analyzed for appropriateness and compliance to clinical guidelines. In the second phase implemented in Q1 2018, a targeted program was initiated to address unindicated transfusions directly: blood bank staff selectively contact providers prior to release of packed cells if the indication is not readily apparent. Following a run-in period, transfusions were again analyzed.
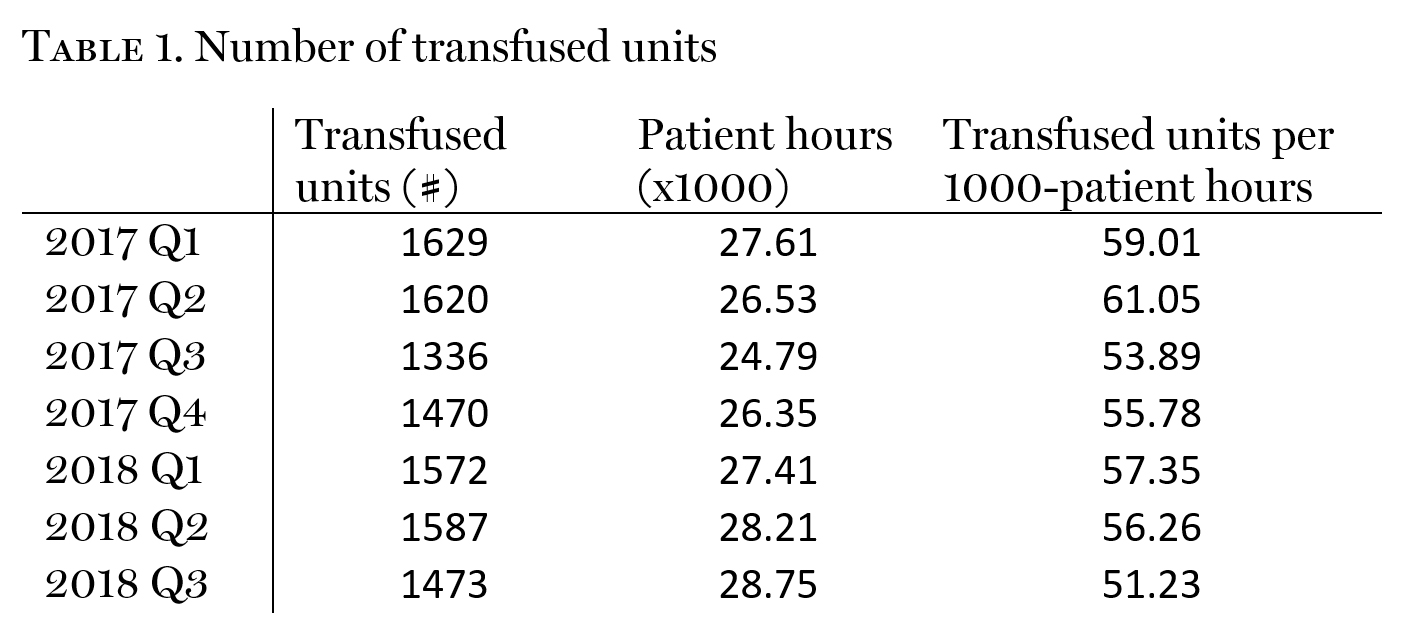
Results: Our overall rate of transfusion decreased from 60.0 to 51.2 units/1000 patient-hours (r=-0.70). This represents an overall 14.7% reduction of blood utilization (Table 1). There was no effect on transfusion-related adverse events (rsq=0.01).
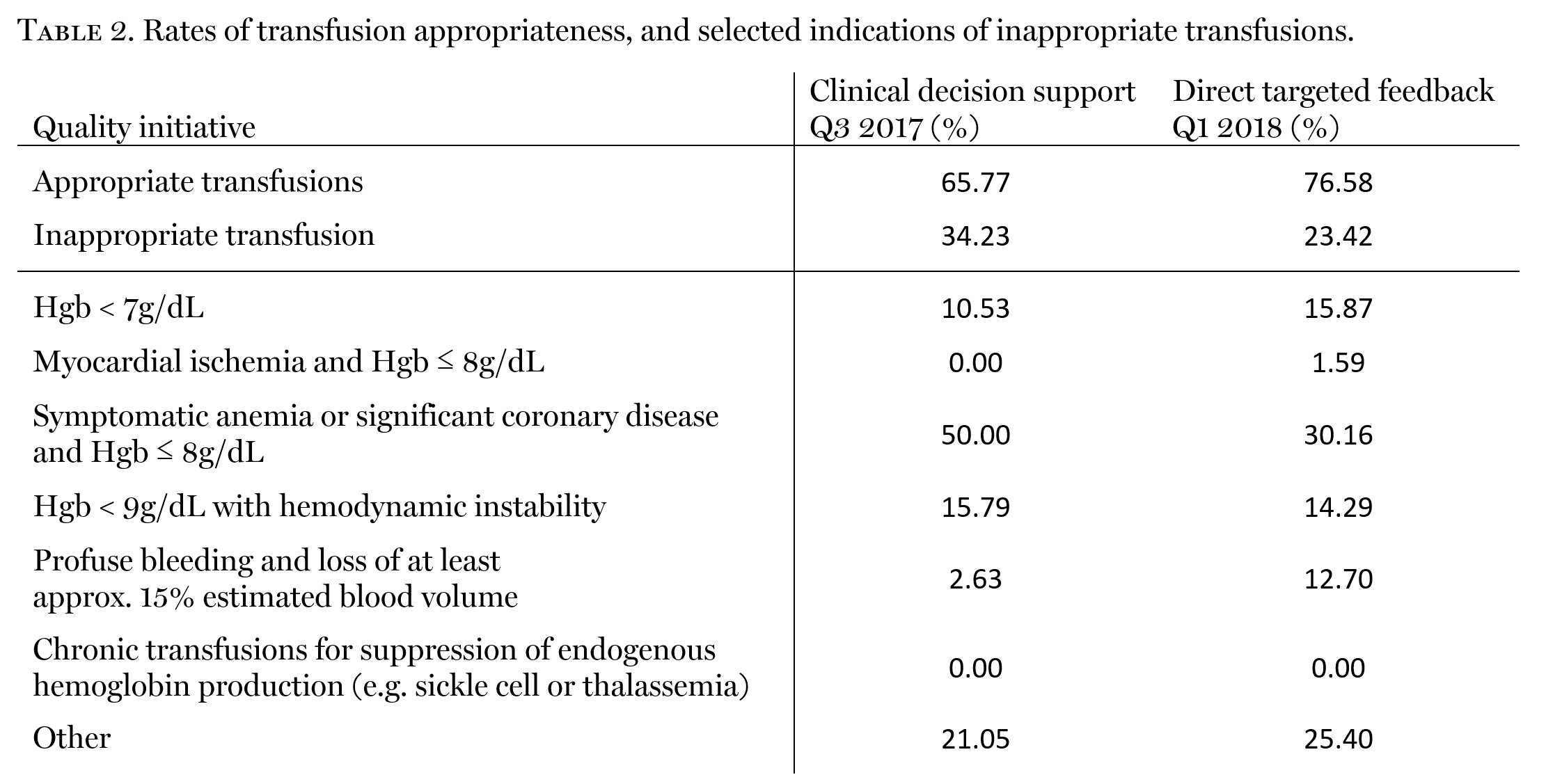
Following the first PDSA cycle, 34.2% of transfusions did not meet guideline-directed indications for transfusion (Table 2). Notably, 79% of these were found to have a pre-transfusion hemoglobin between 7 and 8g/dL; selected indications for these transfusions suggested compliance, signifying hemodynamic instability, symptomatology of anemia, or cardiac instability. Upon close review, however, evidence of these conditions was not readily apparent.
Following a second wash-in period, the proportion of transfusions that did not meet guideline-directed indications for transfusions fell to 23.4%, representing a 10.8% improvement in guideline-compliance. In addition, there was a 19.84% decrease in RBC utilization in the setting of pre-transfusion hemoglobin between 7 and 8g/dL.
Conclusions: Utilizing a PDSA approach, we have decreased our overall RBC utilization by 14.7%, and improved guideline-compliance by 10.8%. We plan to further optimize our blood management program by implementing a revised order set leveraging feedback elicited from blood bank staff during our second PDSA cycle, along with a single-unit transfusion educational campaign. Our goal is to increase transfusion compliance to 80% by Q3 2019.