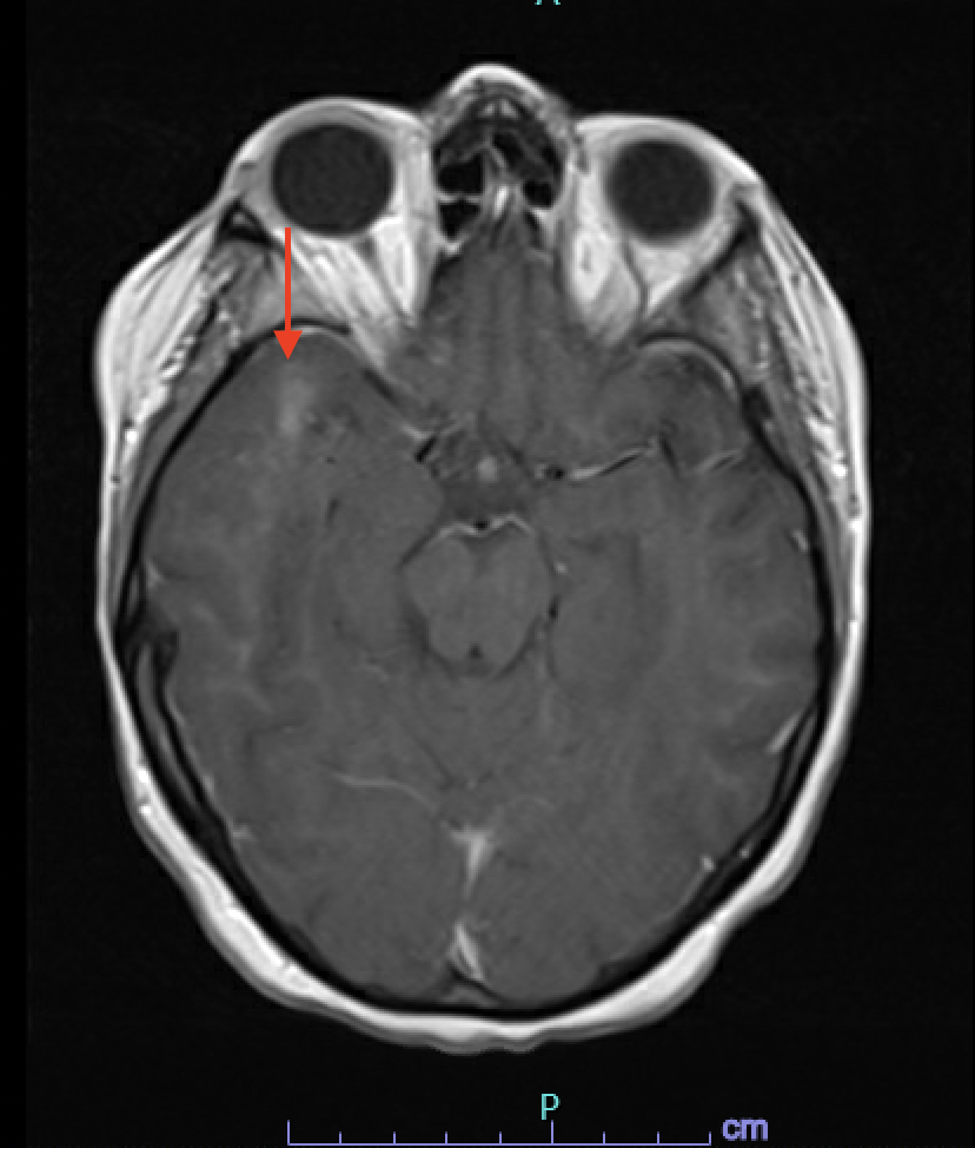
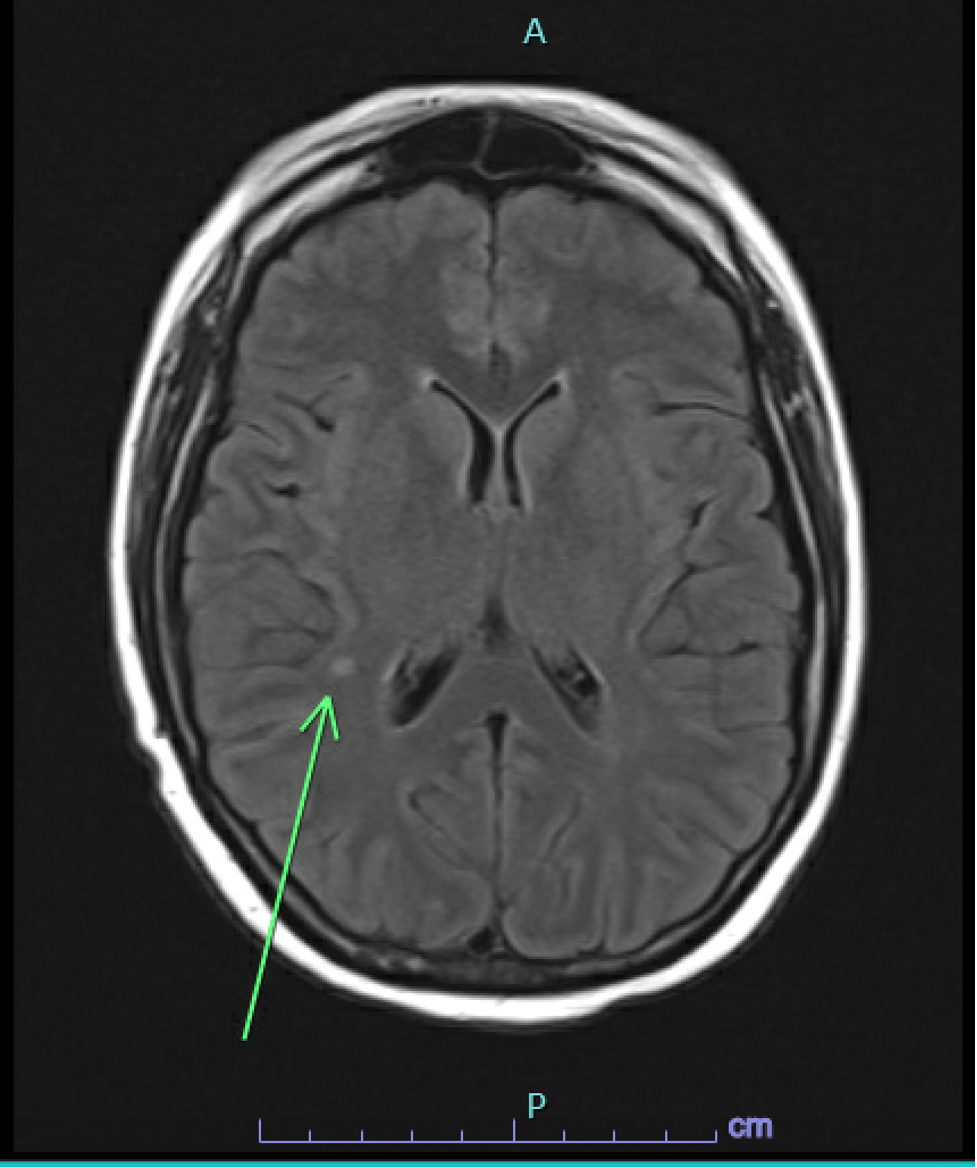
Case Presentation: Our 28-year-old female patient with a history of acute myeloid leukemia (AML) and allogeneic peripheral blood stem cell transplant presented to the Emergency Department (ED) for the evaluation of intermittent confusion and word-finding difficulty for one week. The patient’s vitals on admission were within normal limits. A physical examination was significant for diffuse desquamating rash throughout the skin. Laboratory studies showed a white cell count of 2.87 per microliter, an absolute neutrophil count of 1900 per microliter, hemoglobin 9.7 mg/dl, and a platelet count of 183,000. Further testing was positive for Respiratory Syncytial Virus (RSV), COVID-19, Influenza A, and JC Virus (212,000 copies/ml), followed by the lumbar puncture (LP). LP was consistent with >100M copies of the JC virus and elevated protein levels (i.e., 122 mg/dl). Initially, a CT head was done, which was negative for acute intracranial pathology, followed by an MRI brain, which showed a 4mm T2/FLAIR hyperintense enhancing focus in the right supratentorial region and a right anterior temporal subcortical white matter enhancement measuring up to 14 mm in size with no corresponding FLAIR abnormality suggestive of JCV encephalitis (Image 1&2). The patient’s mental status deteriorated over the course of hospitalization. The patient received virus-specific T cell therapy after enrolling in one of the clinical trials. Despite participating in a clinical trial and receiving virus-specific T-cell therapy, there was only partial improvement in mental status, with no change in viral load or imaging. Subsequently, the patient’s mental status deteriorated, prompting the family to opt for hospice care.
Discussion: The polyomavirus JC virus (JCV), which is recognized for inducing progressive multifocal leukoencephalopathy (PML) in immunocompromised individuals, is discussed here. Oligodendrocytes, kidneys, urothelium, and lymphoid tissues are the primary targets of JCV. During periods of immunosuppression, the non-coding control regions (NCCR) undergo genetic rearrangements that have been implicated in the invasion of glial cells and the development of progressive multifocal leukoencephalopathy (PML). Although previously linked to glial cell infection, recent research suggests that JCV can also affect non-white matter, which complicates the diagnostic process. Infrequent ailments associated with JCV comprise encephalopathy, meningitis, and granule cell neuronopathy. Brain biopsy, JCV DNA PCR, and MRI characteristics are utilized in the diagnostic process. The main goal of treatment is to boost adaptive immunity, and virus-specific T cells are used in some experiments to help with this. Infections caused by JCV, specifically JCV encephalitis in the absence of PML, have an uncertain prognosis; the median survival time for PML is three months. There is potential prognostic value in JCV levels, as a low CSF JCV burden is linked to an extended survival period [1-4].
Conclusions: Our patient is unique in a way that she had a sub-centimeter unifocal subcortical lesion that remained stable on several repeated MRI scans and didn’t have clinical features and imaging typical for PML, which was quite unusual for a typical JCV infection. Monitoring of JCV DNA in CSF is crucial to assessing the therapeutic response of the patient. It also emphasizes that virus-specific T-cell therapy has not shown clinical benefit and more data is required in this regard.