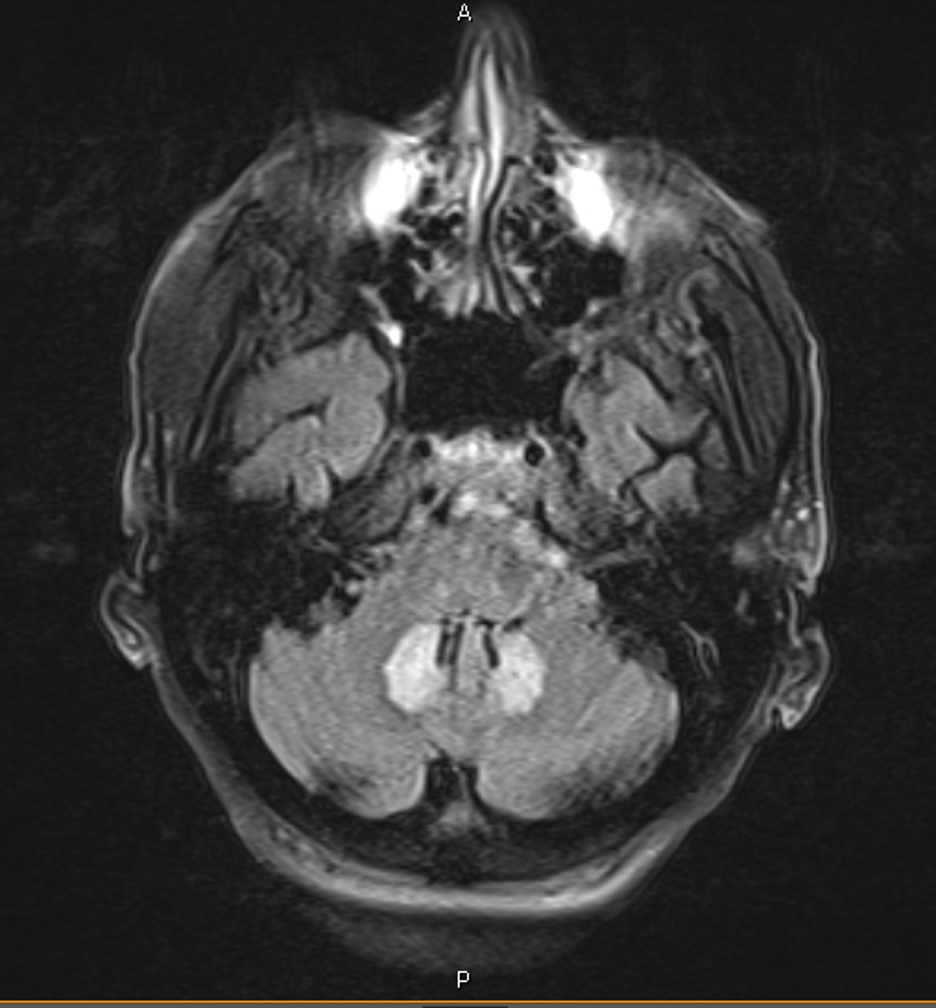
Case Presentation: 66 y.o. male with a past medical history of decompensated liver cirrhosis secondary to hepatitis C virus, IDDM, and a left BKA was admitted for recurrent falls, confusion, and drooling symptoms. He had been taking rifaximin and lactulose. Additionally, he had been started on metronidazole 500 mg 3 times daily 3 months ago to combat recurrent spells of hepatic encephalopathy.Neurologic ExaminationMental Status. Language: abnormal – slowed and slurred speech. CN grossly intact, normal strength in all four extremities. Normal co-ordination. No ataxia in b/l UEs. Ataxia not tested in LE due to L BKA LABS: Normal CBC, CMP, Ammonia 143.CT HEAD normal.Due to his unexplained symptoms, an MRI was obtained revealing findings of FLAIR hyperintensity with restricted diffusion of the dentate nuclei bilaterally Metronidazole was immediately discontinued. Pt was treated with lactulose, rifaximin, and spironolactone. Encephalopathy resolved and the patient was discharged in good condition and remains well.
Discussion: Metronidazole has been used in the prevention of hepatic encephalopathy in patients with cirrhosis, to prevent the production of and the absorption of neurotoxins derived from the gut flora such as ammonia, oxindoles and phenols, mercaptans, and short-chain fatty acids.One of the lesser-known side effects of Metronidazole has been a constellation of CNS symptoms including ataxia, dysarthria, confusion, and seizures, which can result from either short-term or long-term use of the drug. These symptoms have been collectively named “metronidazole induced encephalopathy” (MIE).While the exact mechanism of M.I.E remains unclear, pharmacokinetic studies of the drug have demonstrated that it can cross the blood-brain barrier and causes its direct toxic effects of axonal damage. MRI findings most often demonstrate bilateral involvement of axonal swelling with increased water content, inferring a toxic-metabolic process1,2Although Metronidazole’s primary mode of excretion is renal, up to 50% of the drug undergoes hepatic metabolism2with resultant accumulation of metronidazole and its hepatic metabolites in patients with severe liver dysfunction3Neuroimaging remains the mainstay of diagnosis of MIE, with cerebellum, brainstem, and corpus callosum been identified as the typical sites that are affected. The most remarkable feature of this disease is a complete or near-complete reversal of symptoms and resolution of the original lesions on follow-up MRI, once the drug has been stopped4 however, persistent paresthesia has been documented lasting up to 6 months5
Conclusions: A query of the FDA Adverse Event Reporting System (FAERS) revealed a total of 328 reports of neurotoxicity associated metronidazole since 1991.6 Annually, there are over an estimated 5 million annual prescriptions for metronidazole in the US 7. Therein, 40 cases of neurotoxicity were categorized as life-threatening or considered a contributing factor to patient mortality. As such, the incidence of neurotoxicity is rare, but important. In addition to a detailed history and physical exam, diagnostic neurological imaging can make the diagnosis in this condition. Metronidazole is commonly used by hospitalists. It is therefore extremely prudent to use the smallest doses and shortest possible courses of metronidazole and remains vigilant about monitoring any neurological changes especially in patients with existing liver disease