Background: Wilson Disease (WD) is a known cause of liver test abnormalities. Guidelines have been established to help clinicians avoid routine testing. According to the American Association for the Study of Liver Diseases (AASLD) Guideline, screening for WD—with serum ceruloplasmin, 24-hour urinary copper excretion, and slit-lamp examination for Kayser-Fleischer rings—is warranted in any patient with unexplained liver disease. Despite what the guideline recommends, serum ceruloplasmin is often tested in hospitalized patients with known causes of liver disease. Therefore, we hypothesize that many patients with liver abnormalities are inappropriately tested for WD due to routine testing, resulting in excessive expenditures for our patients and our healthcare system.
Methods: The search terms used to extract patient charts included all discharged patients from an internal medicine service at the University of New Mexico Hospital (UNMH) who had liver abnormalities in 2016-2017. Following the AASLD guideline for WD diagnosis, we determined the number of patients, with explained liver disease, who were inappropriately tested for WD with serum ceruloplasmin. Using data from the UNMH chargemaster and the American Hospital Association (AHA), we conducted statistical analyses to ultimately compute the excessive expenditures that patients and hospitals across the United States (US) incurred for unnecessary testing, with the assumptions that other hospitals have similar practices, as well as comparable admissions for liver abnormalities. Our study has received IRB approval from the University of New Mexico.
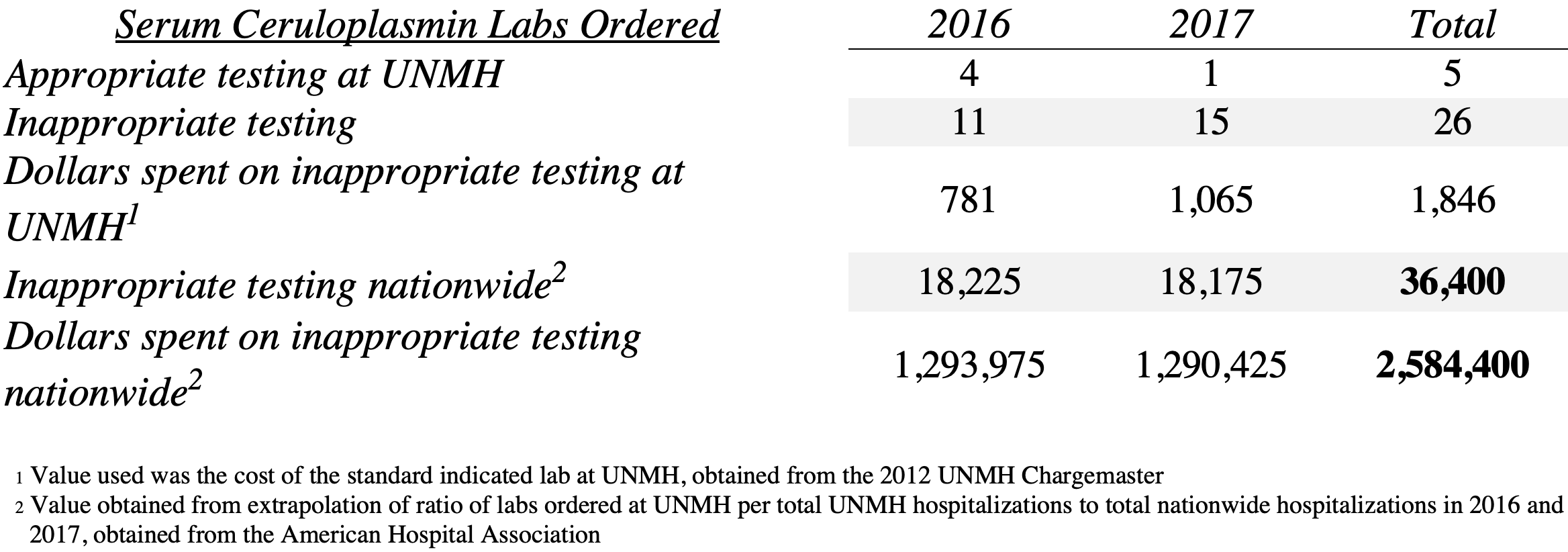
Results: We identified 31 serum ceruloplasmin labs ordered in this cohort of 144 patients. Of those 31 labs, only 5 (16%) were warranted. Overall, this shows that only about 3% of patients should have received this lab test; however, 22% of the total patients received this test. We then extrapolated the same calculations across the US, using the 2016 and 2017 AHA total hospital admissions. Additionally, we calculated the charge of the excessive inappropriate lab work-up both at UNMH and across the US.
Conclusions: Our hypothesis was supported in that 84% of serum ceruloplasmin labs for the patients reviewed were inappropriately ordered, costing our patients and our hospital an extra $1,846 in 2016-2017. Based on the assumptions stated above, we estimate that inappropriately ordered serum ceruloplasmin labs costed patients and hospitals across the US an extra $2,584,400 in 2016-2017. We hope that our findings shed some light on the importance of adhering to established guidelines in order to avoid unnecessary expenditures for our patients and our healthcare system. Future steps include determining the number of liver biopsies with histology that were inappropriately ordered in this cohort of patients.