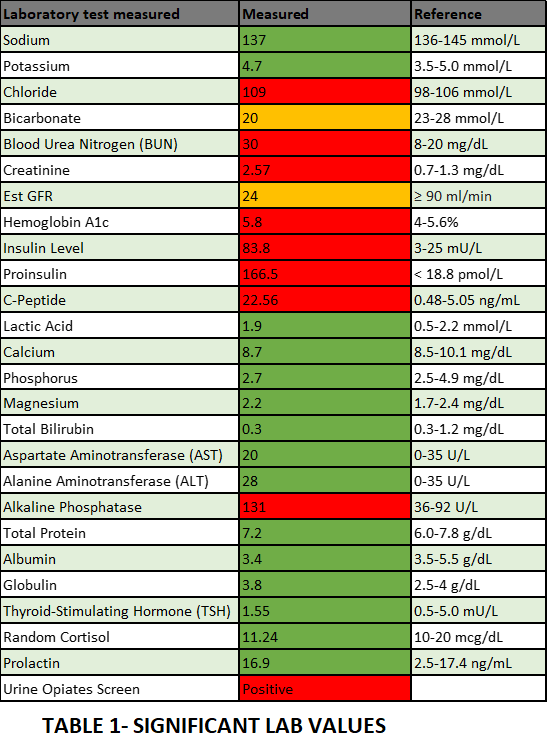
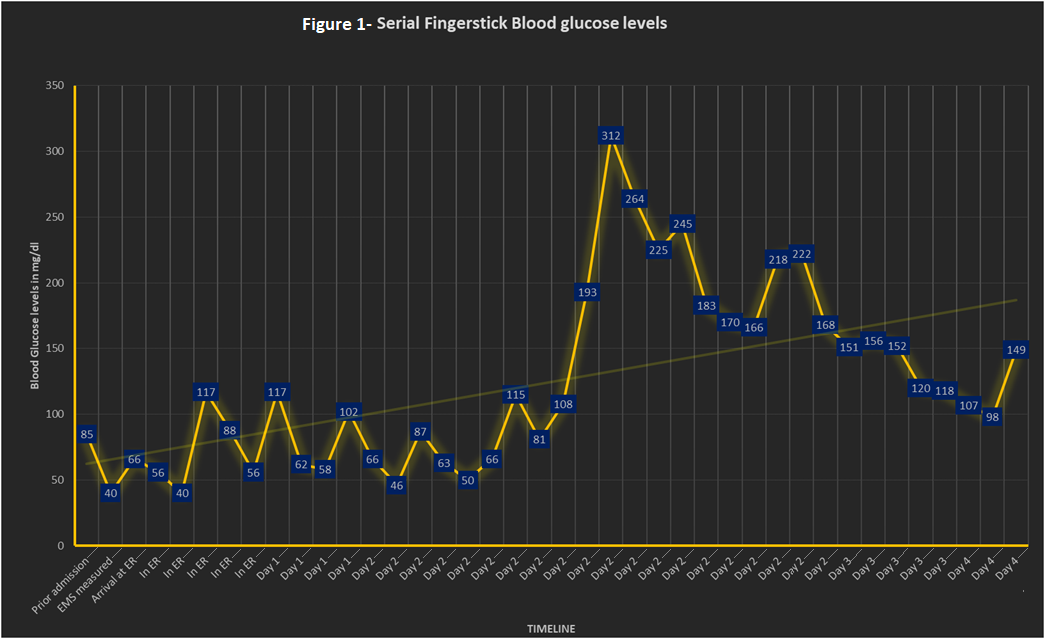
Case Presentation: 73-year-old male had a witnessed fall at home with a minor head injury without loss of consciousness. Later, his wife noted him having new onset convulsive seizures and immediately notified EMS. Upon EMS arrival, he was alert but confused- his fingerstick glucose measured 11 mg/dL. He received oral glucose and was transferred to ED. While in ED, seizures recurred with associated hypoglycemia that were treated with IV lorazepam and IV dextrose. Non-contrast head CT was negative for any hemorrhage or infarct. Prior to arrival, he was lightheaded and confused for the past 7 days after he was started on Trimethoprim-sulfamethoxazole (TMP/SMX) 160/800 mg BID for 7 days by the urologist post cystoscopy for gross hematuria noted since several months; Subsequent biopsy confirmed low-grade-noninvasive papillary urothelial carcinoma. Significant history includes COPD due to smoking, atrial fibrillation on apixaban, CKD IV, MI status post CABG and PCI with stents, pancreatitis status post cholecystectomy. Positive physical exam findings include tremulousness, bilateral rhonchi, 1+ pitting edema. Focused neuro exam revealed no dysarthria, dysphagia or any sensory or motor deficit. Significant lab results (Table.1) include urine toxicology was positive for opiates, normal sulfonylurea levels and high C-peptide, proinsulin and insulin levels. In the ICU, hypoglycemia was managed (Figure 1) with IV Dextrose and IM glucagon with concurrent management of hyperkalemia and atrial fibrillation. CT abdomen/pelvis did not identify any intra-abdominal mass. Neurology consultant advised against anticonvulsive therapy; while the pharmacist identified TMP/SMX use suspecting drug interaction precipitating the seizures in the setting of renal insufficiency. His symptoms gradually improved without any seizure recurrence; he was stepped down to wards and was discharged home. At post-hospitalization follow-up with his PCP, he was neither hypoglycemic nor seizures recurred.
Discussion: Life-threatening adverse effects should be anticipated and/or prevented in patients receiving TMP/SMX by choosing the appropriate dosage. They commonly include severe neutropenia [1], anaphylaxis [2], Stevens-Johnson syndrome and toxic epidermal necrolysis [3]. Hyperkalemia due to blockage of the collecting tubule sodium channel by trimethoprim was also previously reported especially in HIV infected patients [4]. TMP/SMX can cause profound hypoglycemia with concurrent use of sulfonylureas [5]. In the absence of sulfonylurea use, we report a rare case of seizures precipitated by TMP/SMX due to impaired renal clearance resulting in sustained elevation of its levels causing profound hyperinsulinemic hypoglycemia that precipitated seizures. Particularly with the use of IV formulation containing propylene glycol that can cause lactic acidosis [6], the risk of seizure is even higher. While TMP/SMX is a very effective antibiotic, several factors need to be considered before dosing it; these include but not limited to age, history, medication review with drug interaction checks, renal clearance, anticipation and monitoring of adverse effects.
Conclusions: In the setting of renal insufficiency, we propose hyperinsulinemic hypoglycemic seizures as a major life-threatening reaction among the myriad of adverse effects from TMP-SMX, one of the most effective and widely used antibiotic. TMP-SMX is also to be researched in patients on dialysis due to the lack of data in such scenario.