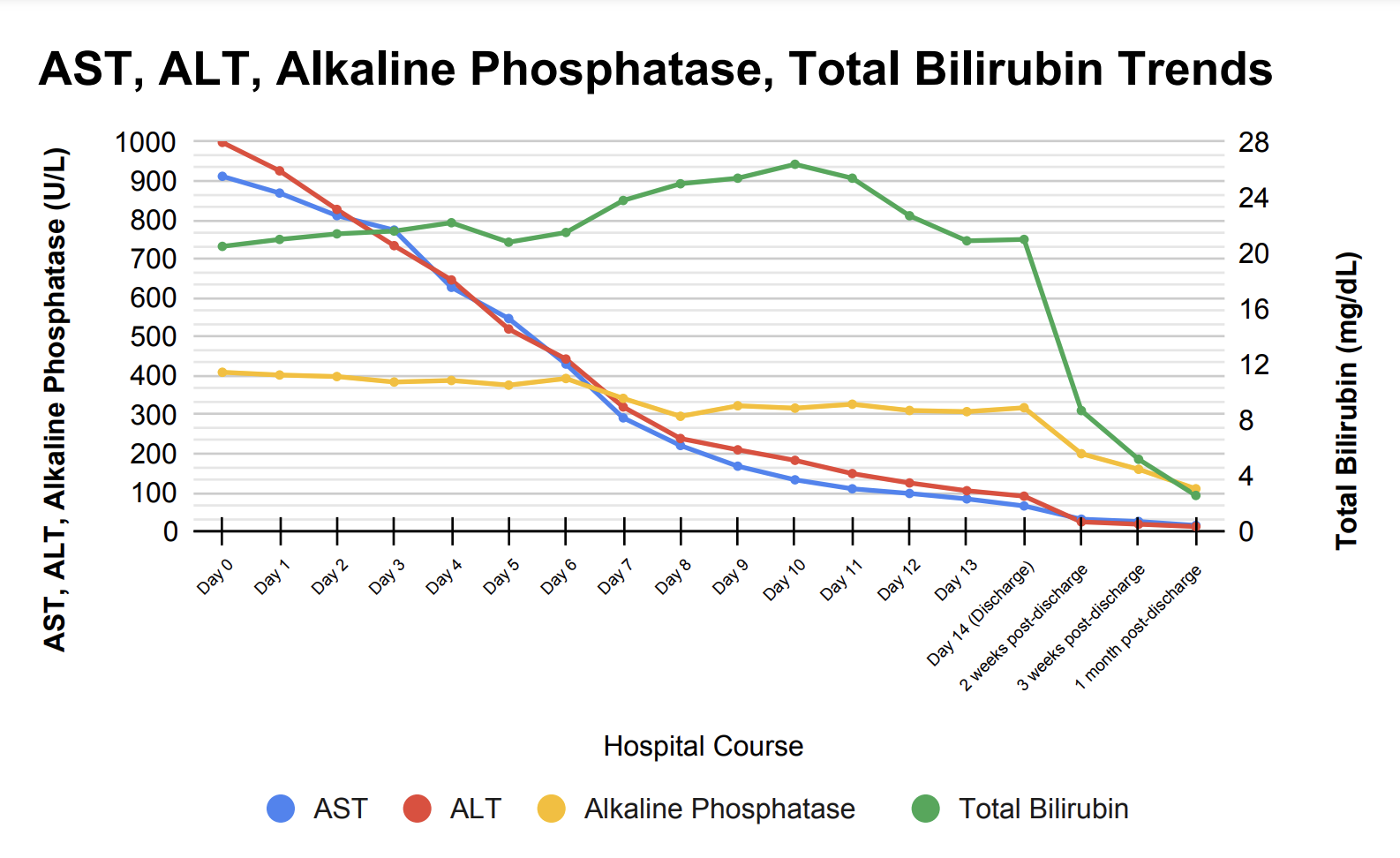
Case Presentation: A 70-year-old female with coronary artery disease (s/p drug-eluting stent x 2 10 years ago), Type 2 Diabetes Mellitus, rheumatoid arthritis, Chronic Obstructive Pulmonary Disease, and remote history of bladder cancer presented to the emergency department with two weeks of nausea, jaundice, dark colored urine, loss of appetite, and clay-colored stool. The patient denied any recent alcohol use or acetaminophen use and had no history of excessive alcohol use. The patient was afebrile and her other vitals were unremarkable. Her physical exam was notable for scleral icterus, bilateral palmar erythema, and jaundice. She had no signs of encephalopathy or ascites, and she was alert and oriented to person, place, and time. Laboratory workup was remarkable for elevated liver transaminases, with AST 912 U/L, ALT 999 U/L, alkaline phosphatase 409 U/L, and total bilirubin 20.5 mg/dL. MRI of the Abdomen showed slightly nodular contour, suggesting cirrhosis, with no suspicious liver lesion. A liver biopsy showed lobular fibrosis with marked inflammatory infiltrate, predominantly lymphomononuclear, and marked lobular disarray in the hepatocytic parenchyma with areas of hepatocytic swelling and scattered acidophil bodies consistent with cholestatic hepatitis. Further laboratory workup showed negative viral and autoimmune serologies. Medication reconciliation revealed that the patient had started using Semaglutide 2mg/3mL injection pens for weight loss and 1500 mg turmeric supplements for rheumatoid arthritis 2 months ago. On admission, the turmeric supplements and Semaglutide were discontinued and supportive care was initiated. During her hospital course, the liver transaminases down-trended, but total bilirubin increased with a peak of 26.4 mg/dL. The patient was discharged once bilirubin levels remained stable, and after discharge, outpatient hepatic panel labs showed continued improvement (Figure 1).
Discussion: Drug-induced liver injury (DILI) is considered a diagnosis of exclusion that is made based on the results of full hepatic workup for other causes of acute liver injury and patient medical history. There have been no published reports of hepatotoxicity due to Semaglutide on LiverTox, while reports of liver injury due to turmeric are rare but have been increasing in association with the usage of higher bioavailability formulations of turmeric supplements. Specifically, the addition of black pepper or piperine in turmeric supplements has been shown to increase the bioavailability of curcumin up to 2000%, precipitating liver toxicity (1). In our patient, the concurrent use of Semaglutide and turmeric may have potentiated each other’s effects through the inhibition of the nuclear factor (NF)-κB signaling pathway and led to further inflammation, contributing to the severity of the DILI in our patient.
Conclusions: This case report adds to the growing number of incidents of turmeric-induced acute liver injury and proposes a possible drug interaction with Semaglutide on the liver. With the growing popularity of turmeric supplements, FDA regulations on HDS should be re-evaluated to ensure that all ingredient combinations and recommended dosages used in turmeric supplements are safe for general consumption. It is also vital for clinicians to conduct a thorough medication review with patients that includes herbal and supplement usage to ensure that HDS-induced liver injury is not overlooked.