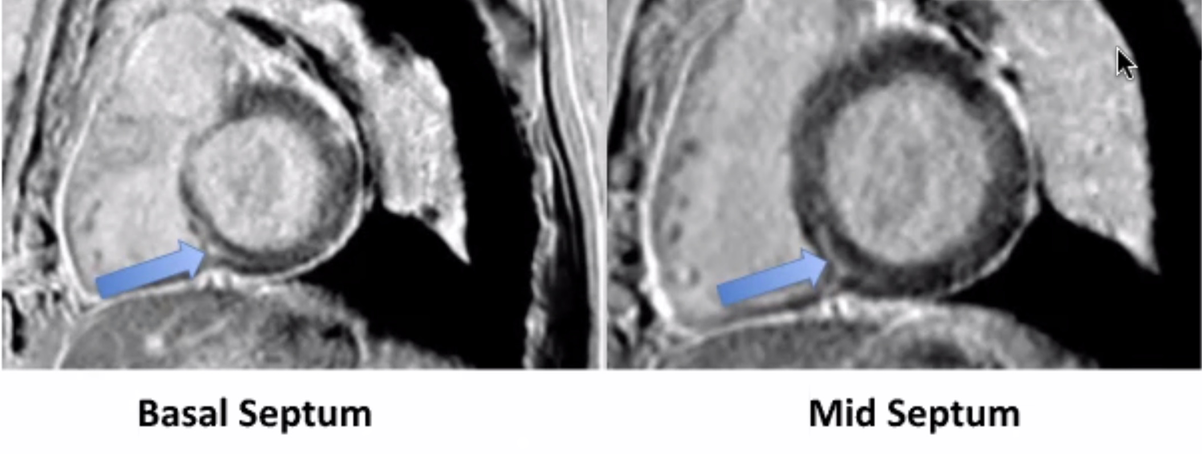
Case Presentation: A 78 year old man with recurrent metastatic adenosquamous lung carcinoma (recently initiated pembrolizumab), CAD s/p CABG, HTN, DM2, and HLD, presented with 2 weeks of fatigue, myalgias, anorexia, and weakness acutely worsening in the prior 2 days. He had no fever, chills, chest pain, dyspnea, orthopnea, palpitations, or lower extremity edema. ED vitals were notable for hypotension at 75/52 (responsive to IV fluids) and tachycardia in the 100s. Labs were notable for troponin I of 7.88, BNP of 1747, Cr of 1.78, and lactic acid of 2.2. EKG showed a new right bundle branch block. TTE showed new mild biventricular dysfunction, LVEF 40-50%. He was given aspirin, started on a heparin drip, and taken emergently to catheterization. Left and right heart catheterization showed patent coronary arteries and normal filling pressures. Endomyocardial biopsy showed lymphocytic infiltrates consistent with acute myocarditis. He was started on 1000 mg daily methylprednisolone and subsequently transitioned to a 60-day prednisone taper. Troponin peaked at 12.04, tachycardia and conduction changes resolved, and his weakness and myalgias improved. When acute kidney injury resolved, cardiac MRI was obtained, revealing patchy myocardial late gadolinium enhancement of the basal/mid septum and epicardial lateral/anterolateral walls consistent with acute myocarditis (Figure 1). He was discharged on day 10 with cardio-oncology clinic follow-up. Clinical vignette patient consent was obtained from the patient and his wife.
Discussion: At time of presentation, the differential included ACS, myocarditis (viral, giant cell, eosinophilic), Takotsubo’s, coronary embolus, and demand ischemia (e.g. from sepsis). Although immune checkpoint inhibitor (ICI) myocarditis usually presents with chest pain and dyspnea, a subset of patients can present with weakness and myalgia. Urgent cardiac catheterization is imperative to rule out MI and obtain endomyocardial biopsy, the gold standard for diagnosing myocarditis. This patient’s first and only pembrolizumab dose was 2 weeks prior to presentation, which is the average time-to-onset. In cases of recent ICI exposure and negative angiography, empiric high-dose corticosteroids are critical while awaiting a definitive diagnosis. T cell activation is promoted by binding of B7 with CD28, but inhibited by binding between cytotoxic T lymphocyte-associated protein-4 (CTLA-4) and programmed cell death protein-1 (PD-1) with B7 and PD-L1 respectively. ICIs promote T cell killing of tumor cells by blocking these inhibitory pathways at CTLA-4, PD-1, or PD-L1. ICIs have dramatically improved survival in several cancers, but can cause immune-related toxicities. Cardiotoxicities such as ICI myocarditis, pericarditis, and vasculitis are not yet widely recognized. Prompt treatment with high dose corticosteroids is imperative, as the mortality can be as high as 55%.
Conclusions: Hospitalists are often the first line providers to raise suspicion of ICI myocarditis and other novel ICI-associated syndromes, prompting cardiac consultation and workup. As yet, there are no specific predictive biomarkers for ICI myocarditis. Prompt recognition will be increasingly important in hospital medicine as the percent of cancer patients eligible for immunotherapy increases.