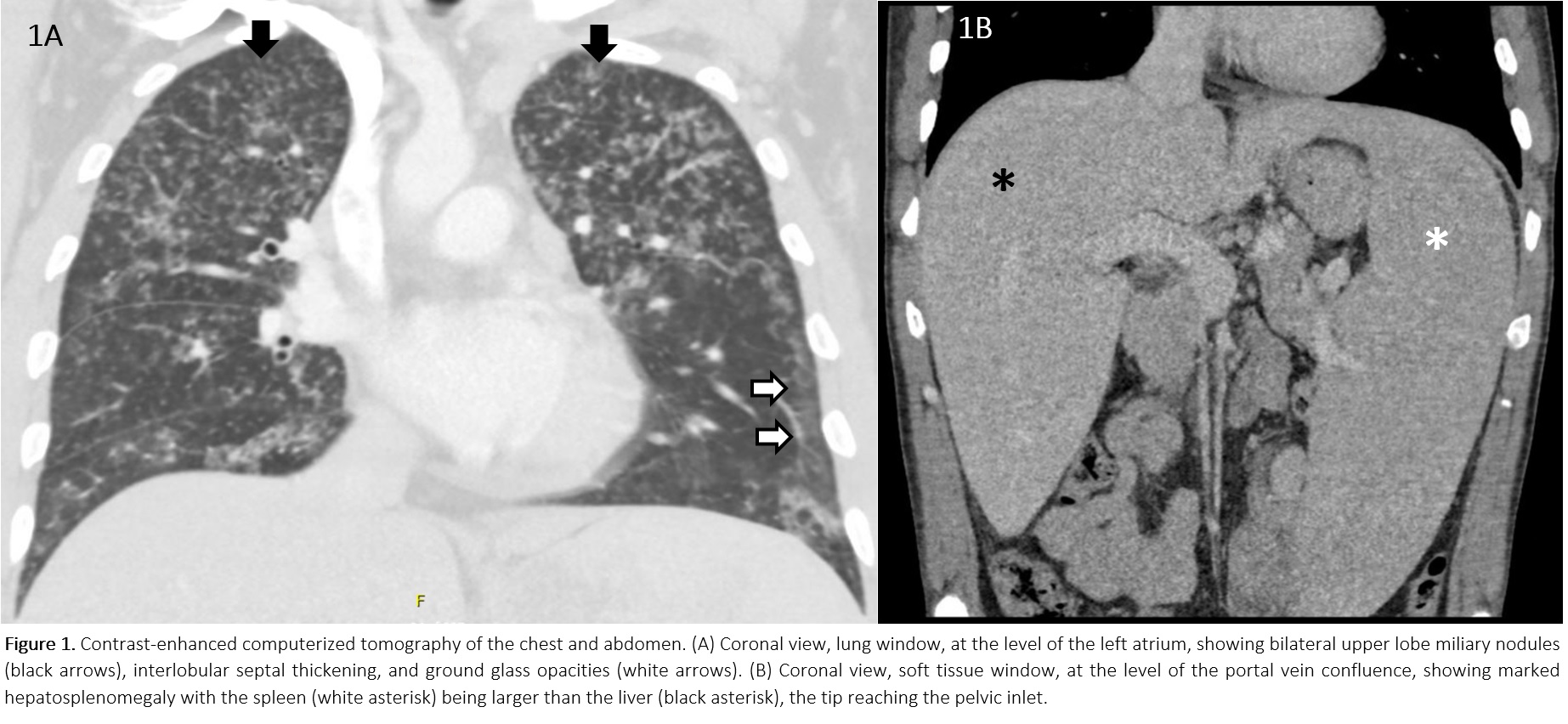
Case Presentation: A 39yo M with no past medical history and taking no medications, presented with 9 months of cough, dyspnea, chills, weight loss, nausea and diarrhea. He had no fever, chest pain, rashes, arthralgias, headaches, abdominal pain, weakness, oral or genital ulcers. He had no recent travel, had no family history of TB or cancer, and had a pet Chihuahua. The symptoms started 2 weeks after a mild COVID infection.He had normal VS, a normal CV exam, scant lung crackles, and hepatosplenomegaly. His WBC count was 44K/mcL, with eosinophils of 30K/mcL. He had a normocytic anemia with a Hb of 8.4 g/dL, and a platelet count of 188K/mcL. CMP was normal. CT imaging of the chest and abdomen (Figure 1) showed bilateral ground glass and consolidative opacities as well as nodular interlobular septal thickening, bilateral upper lobe miliary nodules, and massive hepatosplenomegaly.
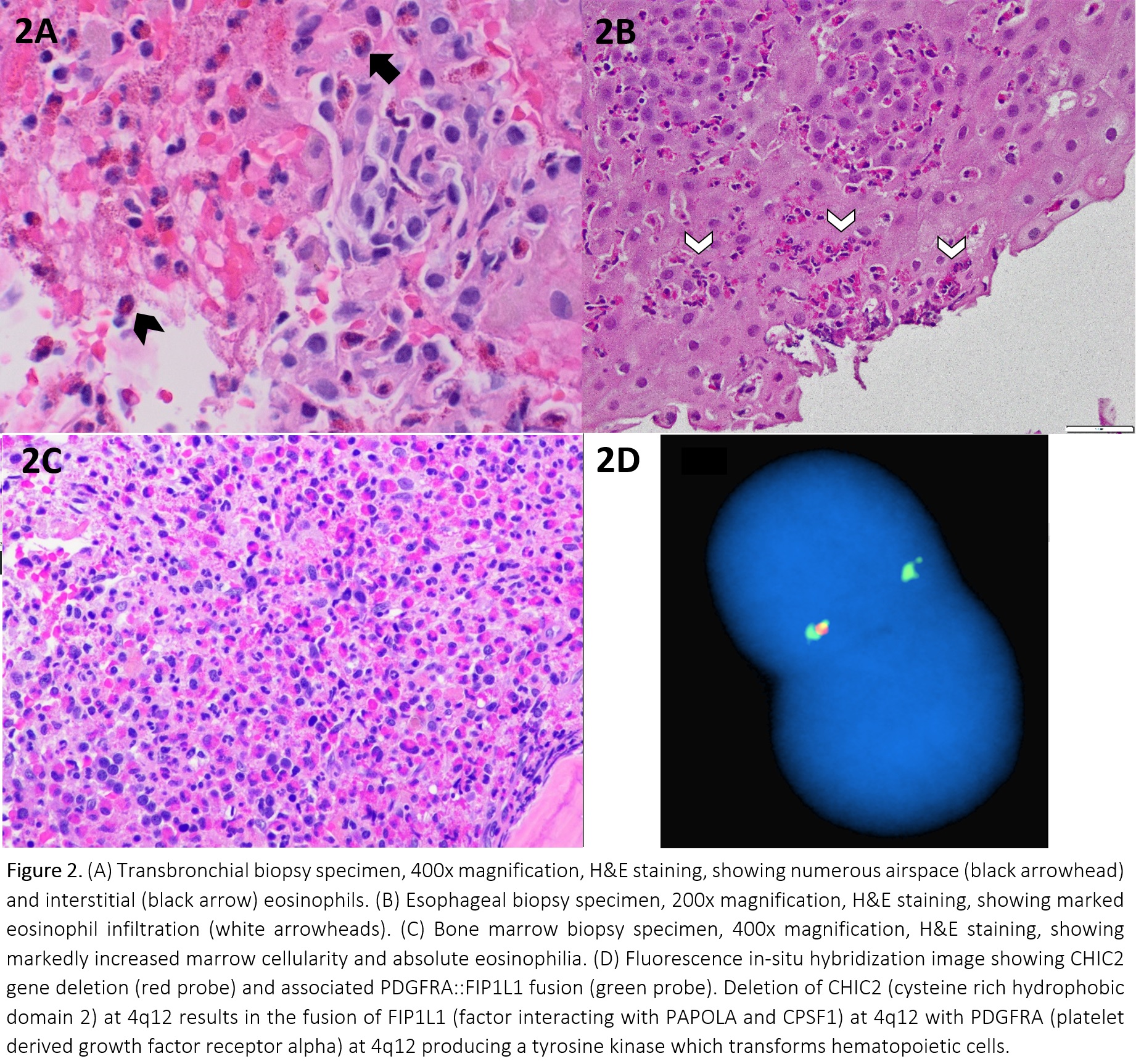
Discussion: Hyperoesinophilic syndrome (HES) is defined as peripheral blood eosinophilia of >1.5K eosinophils/mcL and eosinophil-mediated tissue damage, usually confirmed by documenting tissue hypereosinophilia. Primary HES (M-HES) are clonal disorders, many of which have driver mutations. Secondary HES are polyclonal eosinophil expansions in response to underlying insults that can be infectious, drug-induced, allergic, autoimmune, or secondary to a non-eosinophilic neoplasm (lymphomas). Elevated serum B12 and tryptase have been reported as early specific markers of M-HES in patients with HES, especially in the presence of splenomegaly and unexplained anemia.The patient’s serum B12 and tryptase were markedly elevated. Sputum, serum antigens, serologies, and stool studies were negative for Tuberculosis, Histoplasma, Coccidioides, Aspergillosis, Cryptococcosis, Blastomycosis, Pneumocystis, and Strongyloidiasis. Anti-neutrophil cytoplasmic antibodies were negative. IgE, ACE level and procalcitonin were normal. HIV serology was negative.BAL showed increased eosinophils at 67%, with transbronchial biopsy showing alveolar and interstitial eosinophilia. No viral, fungal, parasitic elements or granulomas were found (Figure 2A). EGD showed patchy mucosal changes, biopsy showed esophagitis with increased eosinophils at >45/hpf (Figure 2B).Peripheral blood smear showed marked eosinophilia. Bone marrow biopsy was hypercellular at >95% with sheets of atypical eosinophils and increased mast cells (Figure 2C) No blasts noted. FISH panel performed on the peripheral blood showed a FIP1L1-PDFRA fusion aberration in 85% of nuclei (Figure 2D), confirming the diagnosis of myeloproliferative variant-hypereosinophilic syndrome. His symptoms improved with 3 days of IV methylprednisolone. He was discharged on oral prednisone and is pending imatinib initiation.The relationship between COVID-19 and eosinophilic disorders is complex, with flares of idiopathic HES and new-onset M-HES reported shortly after infection. A large survey based analysis of 160 HES patients showed that the risk of clinically significant flares after COVID vaccinations was < 1%, but data on the impact of infection on worsening HES is limited to 3 single case reports.
Conclusions: Proper management of HES requires comprehensive evaluation to exclude underlying secondary causes. The finding of elevated serum B12 and tryptase is helpful in identifying M-HES in which further bone marrow, genetic, and flow cytometry studies should be expedited. Larger studies are needed to clarify the relationship between COVID infection and eosinophilic disorders.