Background: Equity is one of the fundamental domains of quality in healthcare. Disparities occur in clinical research enrollment among patients of racial and ethnic minority groups and low socioeconomic status. The racial and ethnic disparities in COVID-19 incidence and mortality accentuates the inequities in access to and representation in clinical trials. In the Adaptive COVID-19 Treatment Trial (ACTT-1), only 20% and 23%of participants enrolled were African Americans and Latinx, while they account for 26% and 33% of COVID-19 hospitalizations in the US, respectively. We sought to understand barriers to COVID-19 clinical research enrollment in a hospital where Latinx and African Americans encompass 68.8% and 17.5% of the COVID-19 population and over 90% of patients are under or uninsured.
Methods: Patients admitted to the geographically isolated COVID care unit at a large urban academic safety-net hospital, were screened by their admitting or primary provider regarding interest in joining the COVID-19 biorepository. Interested participants were then approached by an English or Spanish speaking trained research assistant over the phone or in person for formal consent. The research assistant then documented their enrollment status and any other spontaneously divulged information regarding why they did or did not enroll. We conducted a retrospective analysis of all patients referred for enrollment from August 6, 2020 to November 17, 2020. We categorized each patient into one of the major themes found after referral for biorepository enrollment. Subthemes for those who we were unable to consent or declined to enroll were also identified.
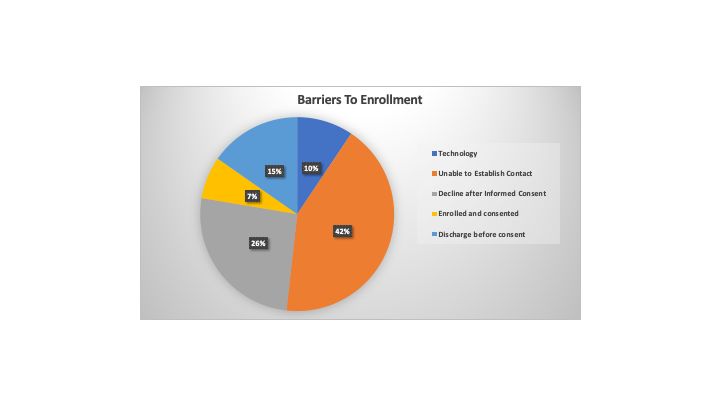
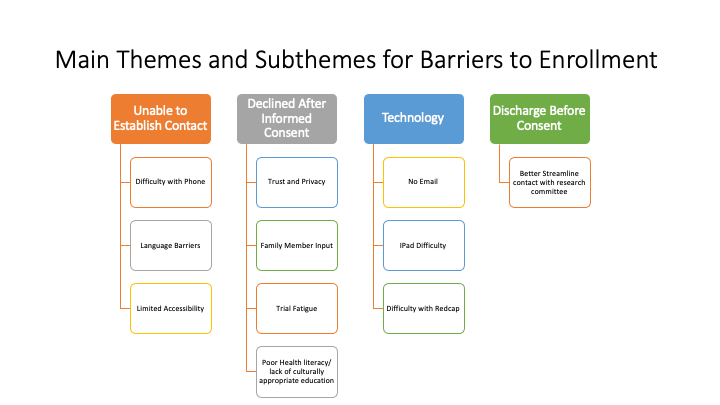
Results: Of the 89 participants screened, 85 (95.5%) agreed to being contacted by a trained research assistant. Only 6 patients (7.1%) agreed to enroll in the COVID-19 biorepository. We categorized the barriers to enrollment for the remaining 79 (92.9%) patients who did not consent (Figure 1). The main barriers included the inability to establish contact (36, 42.4%), discharge before contact attempted (13, 15.3%) and technology obstacles (8, 9.4%). Of the patients who declined after verbal consent was initially provided (22, 25.9%), subthemes volunteered by patients included trust and privacy, trial fatigue, desire for family member input, and inadequate health literacy (Figure 2).
Conclusions: The identification of barriers to enrollment in the COVID-19 biorepository revealed modifiable obstacles to enrolling underrepresented populations. Developing consent tools that do not depend on technology unfamiliar to socioeconomically disadvantaged patients is indicated. Additionally, anticipating how to access patients on an isolated COVID care unit under duress of limited protective equipment is needed. Better coordination with the care team regarding discharge timeline should also increase enrollment. Perhaps most strikingly, patients seemed hesitant to enroll when they were not provided in depth details of how biospecimens will be used in the future, suggesting a lack of trust in science. Investigators may need to craft a more detailed, transparent and culturally competent message. In addition to addressing technical and logistical barriers, trust and understanding between underrepresented and often disenfranchised patients and research personnel must be developed to turn the tide on the epidemic of inequity in clinical research access and representation.