Background: Contemporary treatment for COVID-19 includes the use of SARS-2-CoV direct-acting antivirals (DAAs). An FDA assessment in the Medicare and Veterans Affairs population has suggested that individual agents in this class have significant differences in drug-drug interaction (DDI) potential based on their pharmacokinetic profiles; however, the clinical impact is not well established. We sought to characterize potential DDIs with SARS-2-CoV DAAs while hospitalized for COVID-19 across the pandemic.
Methods: Patients hospitalized with a primary diagnosis of COVID-19 between May 2020 and December 2022 from the PINC AI Healthcare Database were included, assessing medications administered during hospitalization and their potential DDIs with nirmatrelvir/ritonavir, remdesivir, and molnupiravir (per the Emergency Use Authorization factsheet or the package insert). For nirmatrelvir/ritonavir, multiple medications were listed as having potential DDIs categorized as contraindication, avoid concomitant use, or other DDIs (includes recommendation for dose modification, or clinical and laboratory monitoring). For remdesivir, potential DDIs were with chloroquine phosphate and hydroxychloroquine sulfate only. For molnupiravir, no drugs were listed as having potential DDIs.
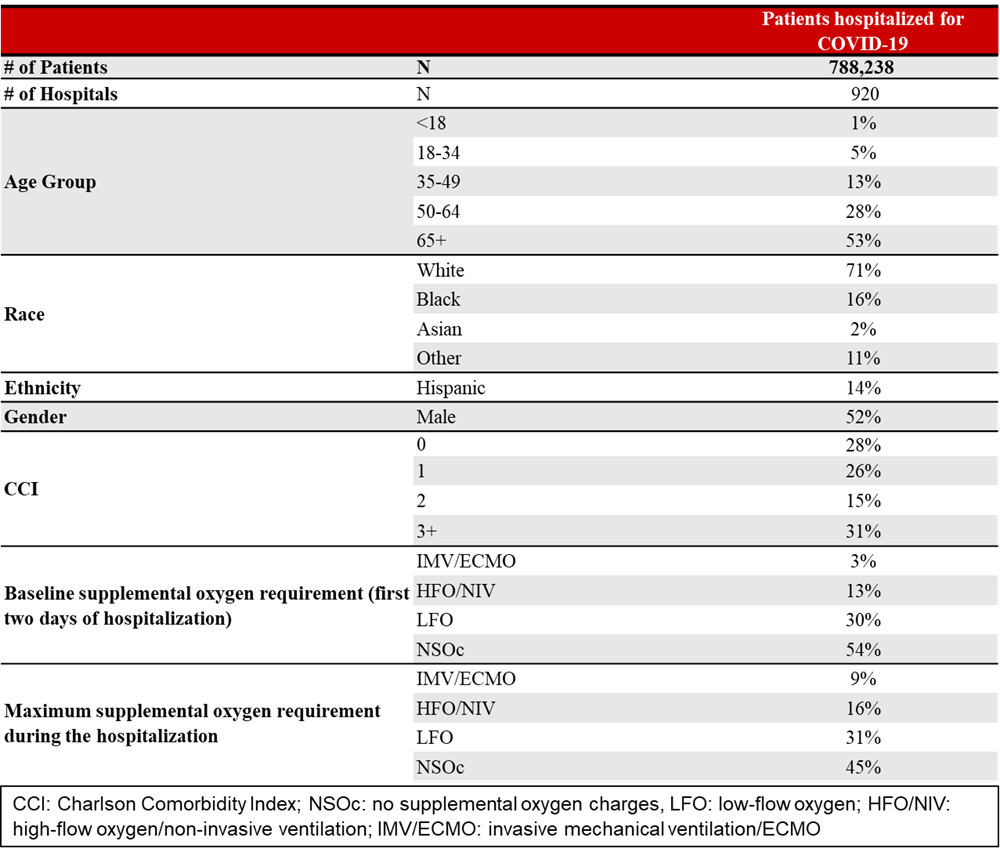
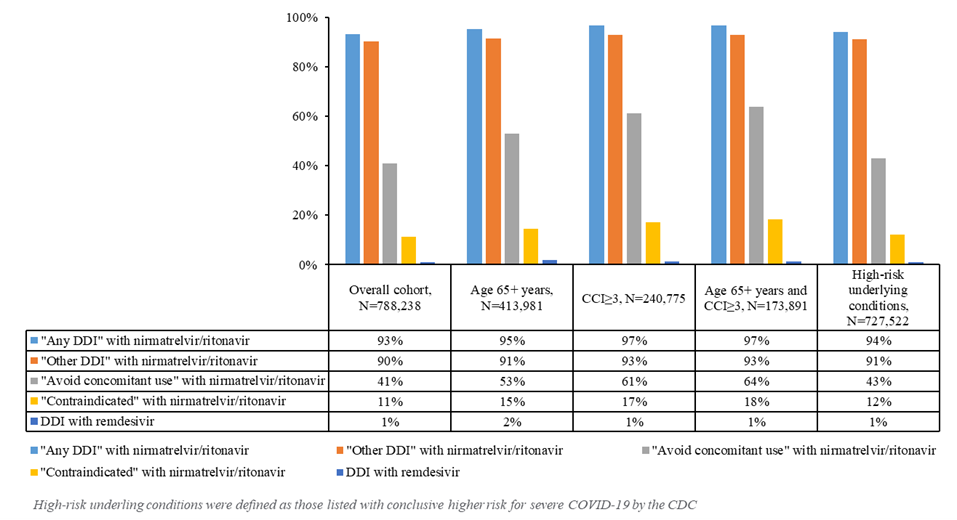
Results: Of the 788,238 patients hospitalized for COVID-19 in 920 hospitals, 53% were 65+ years of age and 31% had Charlson Comorbidity Index (CCI) ≥3 (Table 1). During the study period, 93% would have had any DDI with nirmatrelvir/ritonavir, and in at least 52%, these DDIs would have been clinically problematic (11% “contraindicated,” 41% “avoid concomitant use,” 90% “other DDI”), and the types of interacting medications did not change across the variant periods of the pandemic. The prevalence of “any DDI” was increased in those aged 65+ years (95%), in those with CCI≥3 (97%), and in those with high-risk underlying conditions (94%). The prevalence of “contraindicated” or “avoid concomitant use” also increased in those aged 65+ years (68%), in those with CCI≥3 (78%), and in those with high-risk underlying conditions (55%). The most frequent potentially interacting medications included the following: dexamethasone (67%, “other DDI”), atorvastatin (29%, “avoid”), and amlodipine (20%, “other DDI”).Very few (1%) patients received medications with potential DDIs with remdesivir (Figure 1).
Conclusions: A significant proportion of patients hospitalized for COVID-19 have potential problematic DDIs with DAAs; however, this was predominantly seen with nirmatrelvir/ritonavir while few with remdesivir or none with molnupiravir. These findings emphasize the importance of screening for and considering DDIs when selecting optimal antiviral therapy for COVID-19 treatment.