Background: Semaglutide, a Glucagon-like peptide-1 (GLP-1) agonist, has become one of the most prescribed medications as a result of its beneficial cardiovascular profile and beneficial side effect of weight loss. There is a paucity in literature about its adverse effects. We aimed to conduct a meta-analysis of the severe adverse effects (SAE) of Semaglutide using pooled analysis.
Methods: A systematic search for manuscripts on Pubmed was performed using the terms “Semalutide and adverse effects or side effects.” Initial results included a total of 41 studies. Studies that included children, subjects on multiple antihyperglycemics or did not have a placebo group were removed from consideration. Details of adverse effects were a key inclusion criterion. Statistical analysis was done using Revman software. This study was IRB exempt.
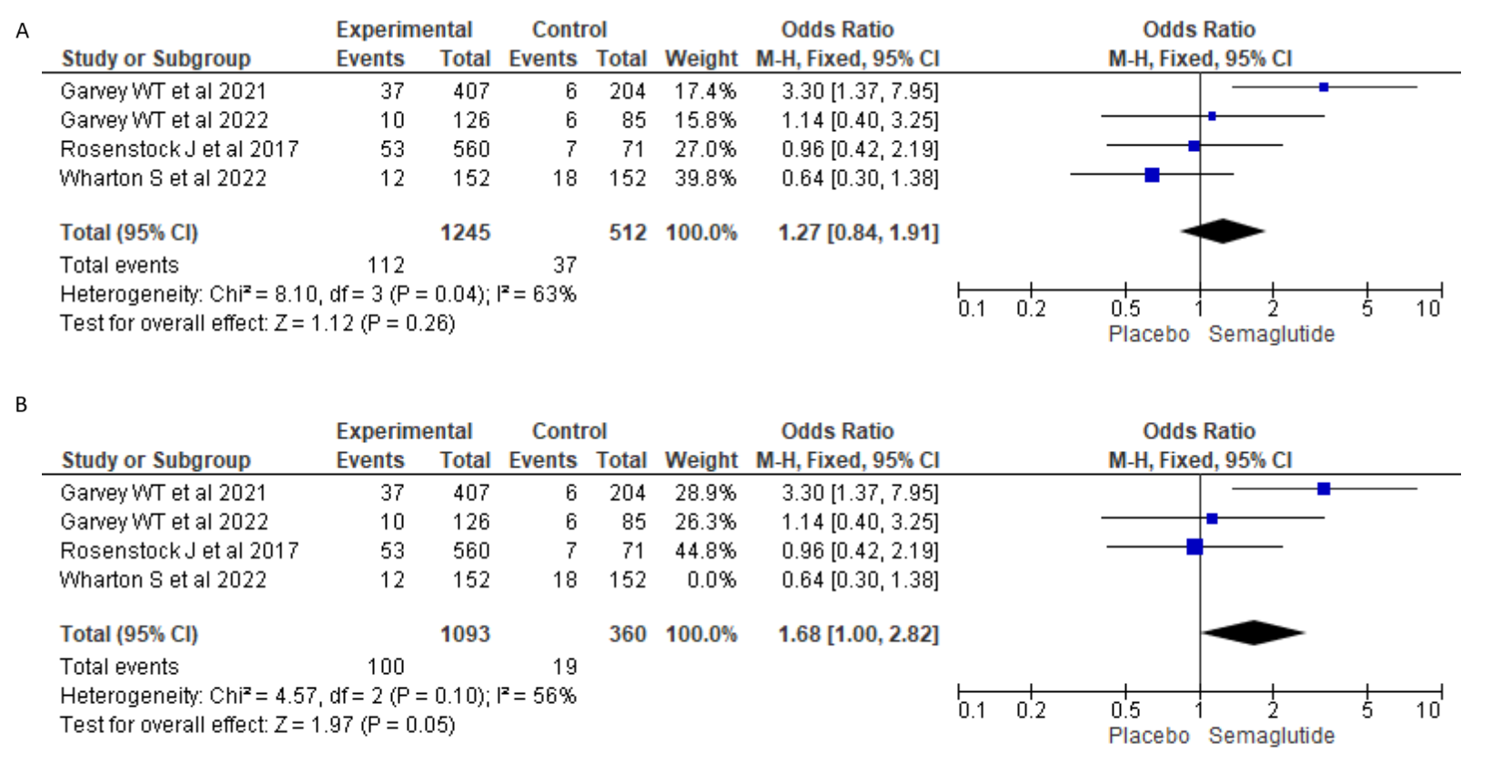
Results: In total 1757 patients from four studies were included in the systematic review and meta-analysis. Of these 1245 subjects received semaglutide while 512 received placebo. The mean duration of trials was about 388 days. Among the participants treated with Semaglutide, the risk of having a SAE was found to be higher, odds ratio (OR)=1.27, but didn’t have statistical significance (95% CI 0.84-1.91), p=0.26, I2=63%). In a sensitivity analysis, after removing the study from Wharton et al, which carried a significant weight in the original analysis (39.8%), the results indicated further increased risk of SAE in the group treated with Semaglutide, OR=1.68 (95% CI 1.0-2.8; p=0.05, I2=56%). It was also noted that 129 (10.3%) participants in Semaglutide had to discontinue due to adverse effects as opposed to 16 (3.1%) taking a placebo. Given that it was not clear if these incidents were included or excluded in each study under SAE, they were excluded from statistical analysis.
Conclusions: Our findings showed that Semaglutide may have increased risk of serious adverse events, but it didn’t reach statistical significance. Some of them are severe enough to discontinue the medication. While the use of medication is increasing exponentially due to beneficial effects, more details of short and long term severe adverse effects needs to be explored.