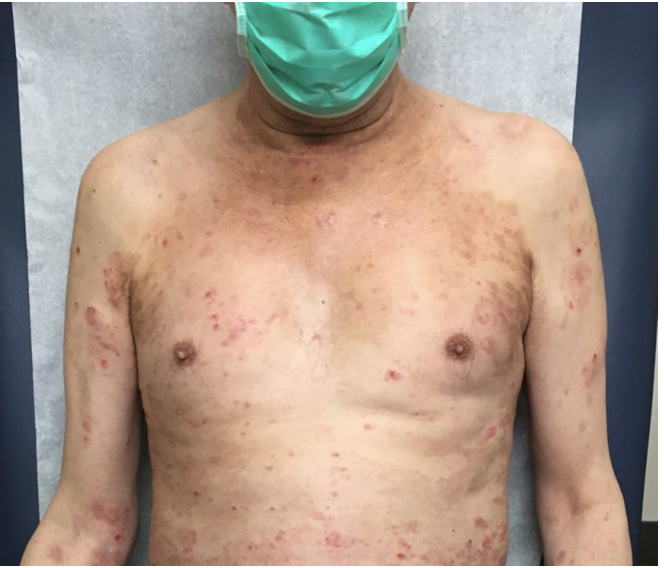
Case Presentation: Our patient is a 66-year-old man, a former 100-pack year smoker, with a history of hypertension, gastroesophageal reflux, emphysema, who was diagnosed in September of 2019 with stage IV squamous cell lung cancer (PD-L1 0%). He was initiated on concurrent radiation with carboplatin and nab-paclitaxel. He had a partial response to this treatment and was initiated on immune checkpoint inhibitor (ICI) durvalumab maintenance as per standard of care. He presented with a vesicular, pruritic rash eight months after initiating this treatment. On exam, his rash was on the right-upper extremity, vesicular, painful, and itchy. Durvalumab was held due to possible immune related adverse event skin toxicity (irAEst). He was prescribed valacyclovir and was started on prednisone 20 mg daily. However, his rash spread from the torso to his feet and extremities (Fig 1). Skin biopsy pathology showed urticarial bullous pemphigoid and DIF positive for C3 and IgG at the basement membrane. Our patient had two hospital admissions for shortness of breath concerning for lung toxicity and for severe immune-mediated skin reaction. Notable labs included eosinophilia, 36.5% eosinophils. He was discharged on high dose prednisone, 80 mg daily (1 mg/kg), with taper and plan to start Dupixent outpatient. A month later, the patient started Dupixent: 600 mg loading dose followed by 300 mg every 2 weeks. Treatment goal was to achieve Grade 1 for skin lesions, which is the level to safely restart durvalumab. A month later the patient’s skin lesions improved. Prednisone was tapered to 30 mg daily and then to 20 mg daily, and he was started on hydrocortisone 2.5% ointment twice daily to affected areas and Clobetasol Propionate 0.05% gel twice daily. Dupixent was terminated 4 months after treatment.
Discussion: Bullous pemphigoid has become increasingly recognized as an irAE of ICIs, including both PD-1 inhibitors (nivolumab, pembrolizumab, cemiplimab) and PD-L1 inhibitors (durvalumab, atezolizumab).[1] Understanding the presenting signs of irAEst will further help to guide care in hospital medicine. A literature search yielded 41 articles that were published between 2015 and 2020 and a total of 58 cases. 87% of cases are DIF positive for C3 and IgG along DEJ. Therapy includes high potency topical corticosteroids, systemic corticosteroids, doxycycline + niacinamide (for maintenance), and rituximab, omalizumab, methotrexate and discontinuation of immunotherapy. ASCO guidelines for work up include dermatologic referral and skin biopsy. For management, the American Society of Clinical Oncology (ASCO) recommends continuation of ICIs for blisters < 10% body surface area and for grade 2 toxicities or above (once a blister is deroofed).[2] Recommended treatment includes high-potency topical corticosteroid, such as prednisone 0.5 - 1 mg/kg with taper over 4 weeks. For grade 3 toxicities, ICIs should be held, 1 to 2 mg/kg IV methylprednisolone initiated with at least a 4-week taper.
Conclusions: Dupixent is not currently recommended in the ASCO guidelines. It is a monoclonal antibody which received FDA approval for use for moderate-to-severe atopic dermatitis in 2017 and for asthma in 2018. It binds to the alpha subunit of interleukin-4 receptor, modulating the signaling of both interleukin 4 and interleukin 13 pathways.[3] We propose that adding Dupixent to corticosteroids can provide faster resolution and is an effective adjunctive therapy with corticosteroids for Grade 3 and above skin toxicities.