Case Presentation: A 71-year-old woman with poorly differentiated squamous cell carcinoma (SCCA) of the left cheek with recent initiation of nivolumab therapy presented to the Emergency Department with five days of bilateral thigh pain, generalized weakness, and dyspnea.
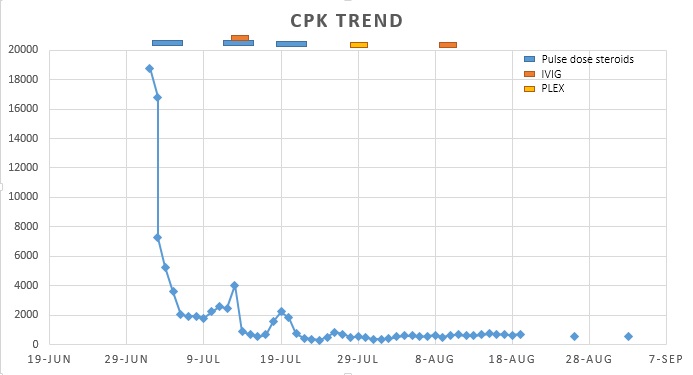
The exam was remarkable for tachycardia, hypotension, tachypnea as well as neck and proximal muscle strength weakness. EKG demonstrated arrhythmias varying from sinus tachycardia with alternating bundle branch blocks to accelerated idioventricular rhythm. Labs were remarkable for CPK 18,760, AST 1100, ALT 538, TSH 40 with FT4 6 and a troponin of 8.3.
The patient necessitated mechanical ventilation on the evening of admission. A myositis panel was unrevealing. MRI brain and C-spine demonstrated no significant disease.
Consequently, the patient was diagnosed with PD1 inhibitor-induced toxicity leading to inflammatory myositis, myocarditis, acute liver injury, and autoimmune hypothyroidism. Over the course of 2 months, she was treated with mycophenolate mofetil, multiple courses of pulse dose steroids with IVIG, and a course of plasmapheresis. The patient’s exam and CPK levels improved and she was discharged to a long-term acute care hospital for ventilator management with monthly IVIG and a steroid taper.
Discussion: Despite PD1 inhibitor’s significant clinical efficacy, it has been associated with a broad spectrum of immune-related adverse events (iRAEs), affecting almost all tissues and organs (2)(3). Severe neurologic iRAEs involving the central or peripheral nervous system (such as myositis) are rare, occurring in 0.1 to 0.8% of patients treated with PD1 inhibitors (6), with only two reported cases requiring intubation (1).
Our patient developed multiple concomitant toxicities including hepatitis, thyroiditis, and myocarditis, associated with a higher mortality rate (4). Prompt recognition of immune-related myositis (irMyositis) is critical as treatment with immune-modulating therapies can reverse life-threatening complications.
Clinical manifestations of irMyositis predominantly include myalgia with weakness in an axial and limb-girdle pattern as well as oculomotor weakness (not present in our patient), elevated CPK levels, and a negative myositis panel (4). Muscle biopsy may help in establishing the diagnosis and also to rule out other neuromuscular diseases.
A series of cases report effective treatment with steroids as first-line treatment, but if refractory, IVIG and/or plasmapheresis should be considered (1).
Conclusions: As PD1 inhibitor use becomes more prevalent so do cases of its toxicity. Prompt recognition and treatment is of paramount importance to address life-threatening complications.
1. Liewluck T, Kao JC, Mauermann ML. PD-1 Inhibitor-associated Myopathies. J Immunother. 2018;41(4):208–11.
2. Al-kindi SG, Oliveira GH. Reporting of immune checkpoint inhibitor- associated myocarditis. Lancet. 2018;392(10145):382–3.
3. Anquetil C, Salem J-E, Lebrun-Vignes B, Johnson DB, Mammen AL, Stenzel W, et al. Immune Checkpoint Inhibitor–Associated Myositis. Circulation. 2018; 138(7):743-745
4. Johnson DB, et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med. 2016;375(18):1749–55.
5. Touat M, et al. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology. 2018;91(10):10.
6. Cuzzubbo S, et al. Neurological adverse events associated with immune checkpoint inhibitors : Review. Eur J Cancer. 2017;73:1–8.
.jpg)