Background: Direct acting oral anticoagulants (DOACs) have been approved for conditions such as non-valvular atrial fibrillation and venous thromboembolism. High quality randomized controlled trials have demonstrated the safety and efficacy of DOACs as compared to warfarin with exceptions in specific patient populations (such as those with mechanical heart valves). DOACs are more convenient and generally maintain steady state at fixed doses. Despite the evidence and convenience of DOACs, many patients with standard DOAC indications are still prescribed warfarin.
Purpose: We launched a quality improvement initiative to identify patients prescribed warfarin who might be eligible to transition to DOACs. In designing our intervention, we sought to identify the most common barriers to DOACs transition, and to educate providers about standard DOAC indications and benefits.
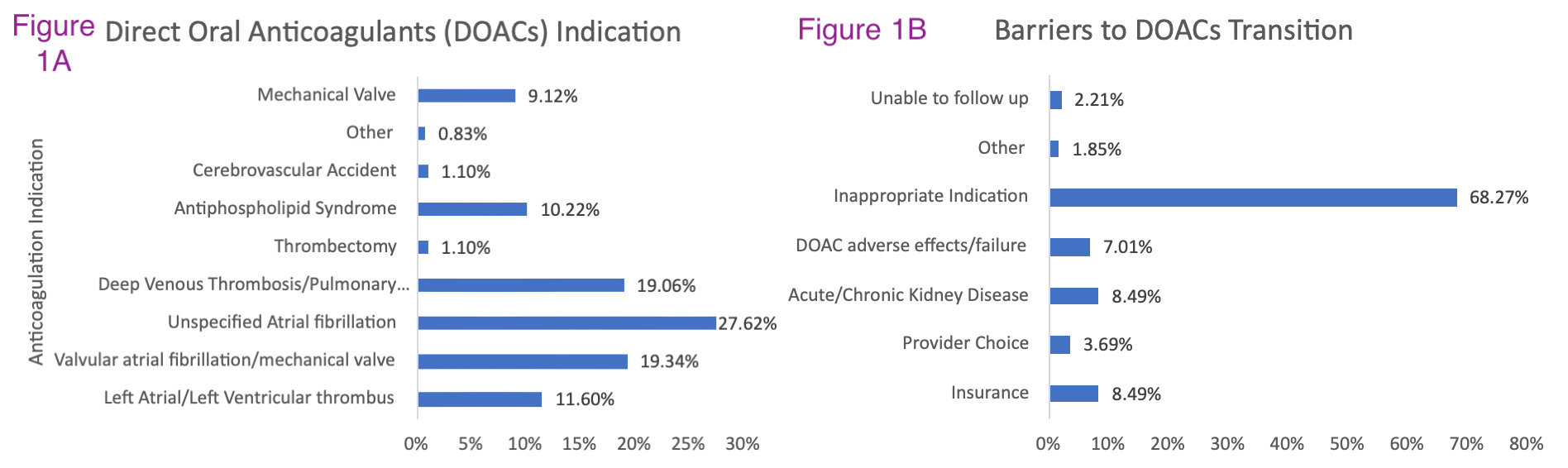
Description: We first identified current inpatients at our tertiary center who were prescribed warfarin for a long-term condition with no obvious contraindication to a DOAC such as end stage renal disease. We did a baseline data pull to compare the relative safety of DOACs compared to Warfarin in terms of 30-day readmission complications. Our team consisted of 3 pharmacists, 5 internal medicine residents, and 2 supervising attending physicians. The pharmacy first identified current inpatients on warfarin and conducted an initial screen for DOAC eligibility. Internal medicine residents then contacted primary providers to inquire about DOAC transition. If the primary team agreed with DOAC transition, our team would provide dosing guidance and follow the patient into the post-discharge period to ensure successful transition. Follow up surveys were conducted to measure convenience and satisfaction with DOACs, or conversely to identify any unexpected barriers that prevented DOAC transition. Barriers were recorded in all cases in which patients were inappropriate for DOAC transition. Of 344 patients evaluated in 2021-2023, 49.7% had standard indications for DOAC with unspecified atrial fibrillation (27.6%) being the most common (Figure 1a). Common barriers for transition were non-standard indications (68.27%), insurance/cost (8.49%), kidney disease (8.49%), DOAC adverse effects/failure (7.01%), and provider preference (3.69%) [Figure 1B]. Overall, 8.2% of patients were successfully transitioned from Warfarin to DOACs.
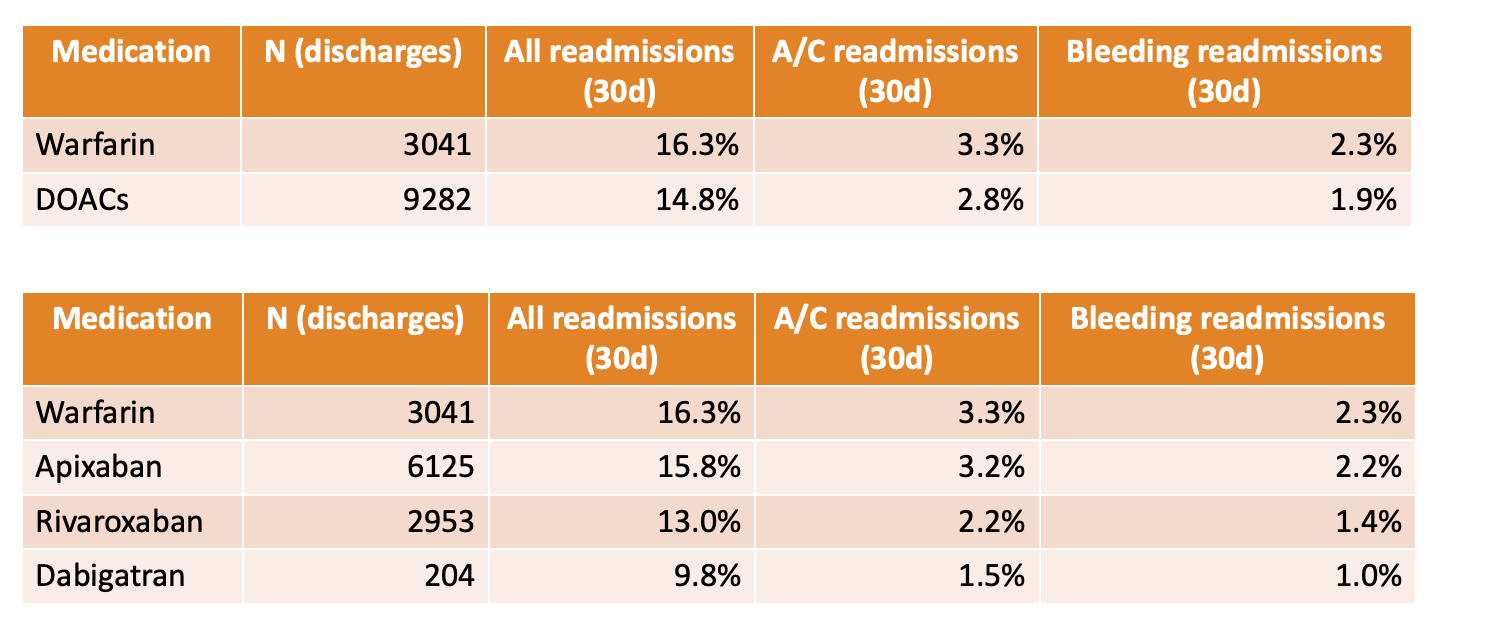
Conclusions: In conclusion, we were able to identify many patients on Warfarin therapy who were eligible for transition to DOACs. Since the most common barrier was the existence of non-standard indications, these data suggest that at our center, we have successfully transitioned many patients to DOACs already. We identified certain patient populations that may represent an opportunity for additional DOAC transitions, such as patients with documented misdiagnosis of valvular atrial fibrillation not supported by clinical data. We also identified cost as a major barrier suggesting a potential role for collaboration between our center and payors for patient assistance programs to ensure equity of care. In a related analysis, we discovered that historically at our center, patients on DOACs had reduced rates of all-cause (14.8% versus 16.3%), thrombosis-related (2.8 vs 3.3%), and bleeding-related 30-day readmissions (1.9 vs 2.3%) [Figure 2]. In the next phase of our intervention, we plan to evaluate whether our success in transitioning patients to DOACs has enhanced our prevention of these readmission complications.