Background: Revefenacin (REV), a once-daily, long-acting muscarinic antagonist for nebulized inhalation, was recently approved for maintenance treatment of chronic obstructive pulmonary disease (COPD). We present post hoc efficacy and safety data from three phase 3 trials in patients with moderate to very severe COPD by patient subgroup (<65 y, 65–75 y, >75 y).
Methods: This post hoc study analyzed data from the 12-week 0126 and 0127 and 52-week 0128 phase 3 studies. Patients in the 0126 and 0127 trials received REV 88 μg, REV 175 μg, or placebo (PBO) once daily (QD). Patients in the 0128 study received REV 88 µg, REV 175 µg, or tiotropium (TIO) 18 µg QD. Efficacy endpoints included the least squares (LS) mean changes of trough forced expiratory volume in 1 second (FEV1) from baseline to day 85 (0126, 0127) and day 365 (0128). Safety endpoints (0126, 0127, and 0128) included the number of patients who experienced adverse events (AEs), serious AEs, deaths, AEs leading to permanent discontinuation or interruption, and most frequently reported AEs and serious AEs.
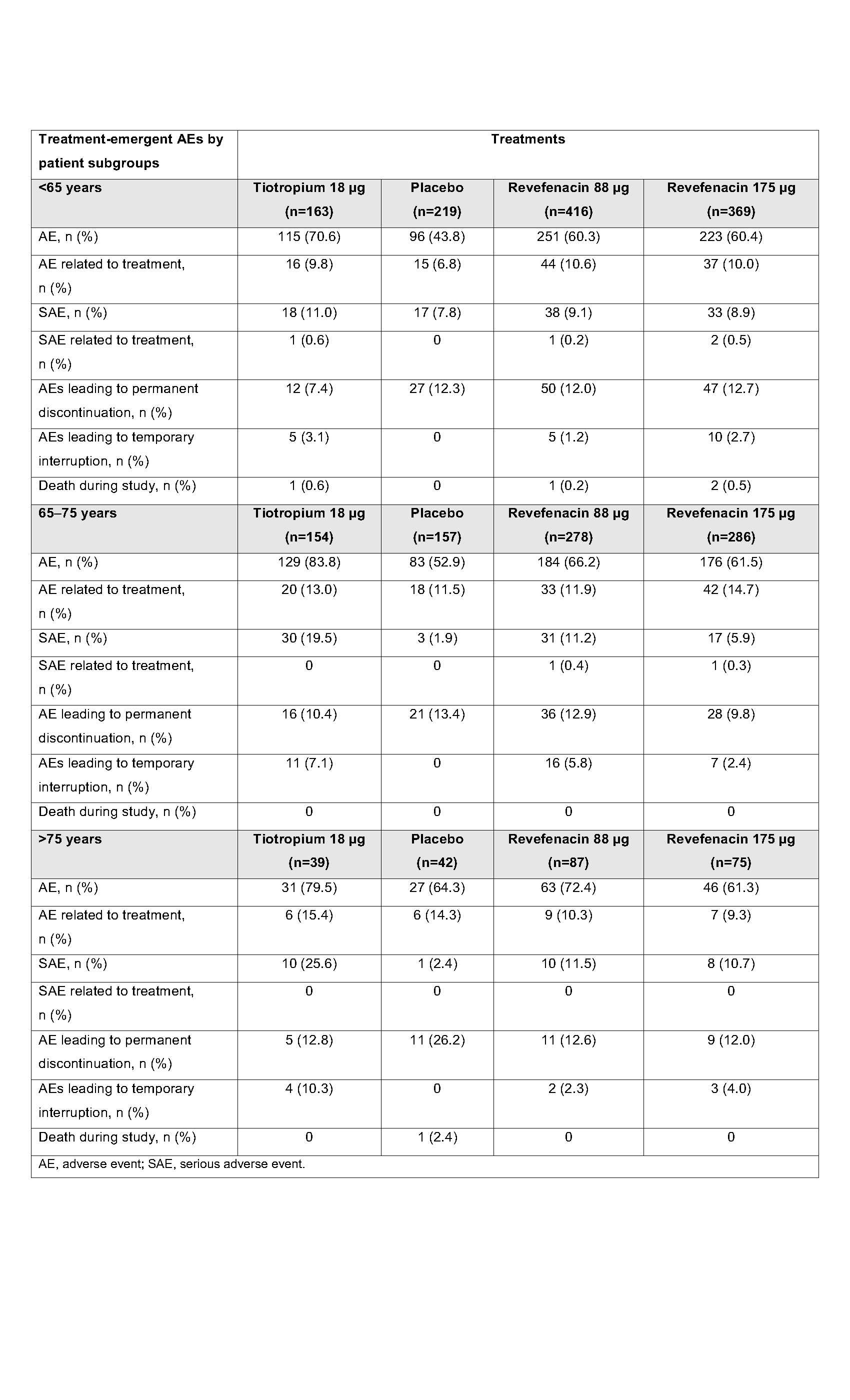
Results: Across both the 65–75 y and >75 y subgroups in 0126 and 0127, significantly greater LS mean increases in trough FEV1 were observed at day 85 with REV 88 µg (86.0 mL, 88.2 mL, respectively) and REV 175 µg (133.3 mL, 105.3 mL) vs PBO (−38.7 mL, −35.1 mL). Significant differences were not observed with REV 88 µg and REV 175 µg vs PBO in the <65 y subgroup. Significant differences were observed for the <65 y subgroup in 0128 in trough FEV1 from baseline at day 365 with REV 88 µg (61.3 mL), REV 175 µg (66.6 mL), and TIO 18 µg (91.2 mL). Significant differences in trough FEV1 from baseline at day 365 were not observed for all treatments for the 65–75 y and >75 y subgroups. The safety profiles of REV (88 µg and 175 µg) and TIO 18 µg were generally similar and consistent among all subgroups over 52 weeks. Patients who received PBO experienced fewer AEs across the <65 y and 65–75 y subgroups. In the >75 y subgroup, patients who received REV 175 µg experienced fewer AEs (Table). The most frequently reported AE (%) in the <65 y, 65–75 y, and >75 y subgroups was worsening or exacerbation of COPD for those who received REV 88 µg (18.3, 19.4, 21.8, respectively), REV 175 µg (16.5, 14.7, 16.0), TIO (27.0, 29.2, 28.2), and PBO (10.0, 10.2, 23.8). Serious AEs were comparable between treatments across subgroups, and mortality was low (Table).
Conclusions: Appreciating the post hoc nature of the analysis, this study showed that REV (88 µg and 175 µg) and TIO provided consistent benefits in lung function and had generally similar and consistent safety profiles among all patient subgroups.