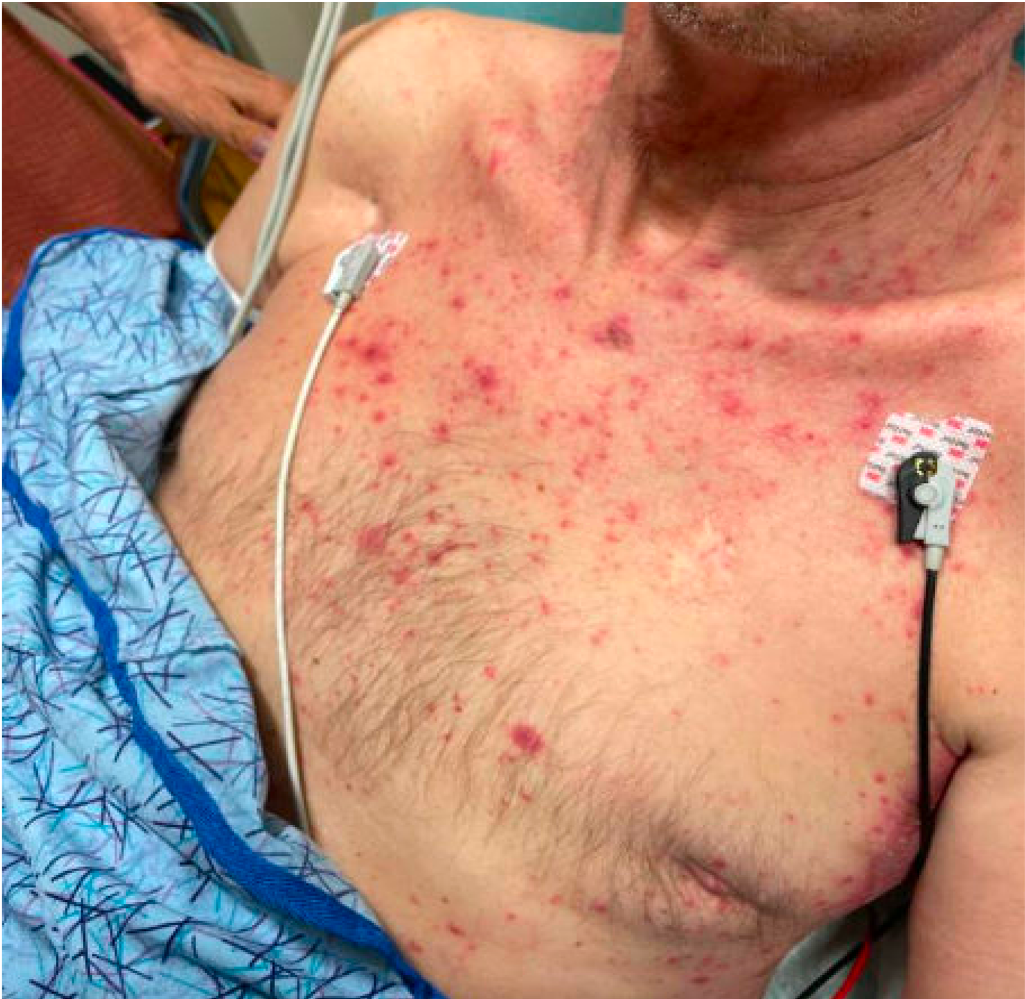
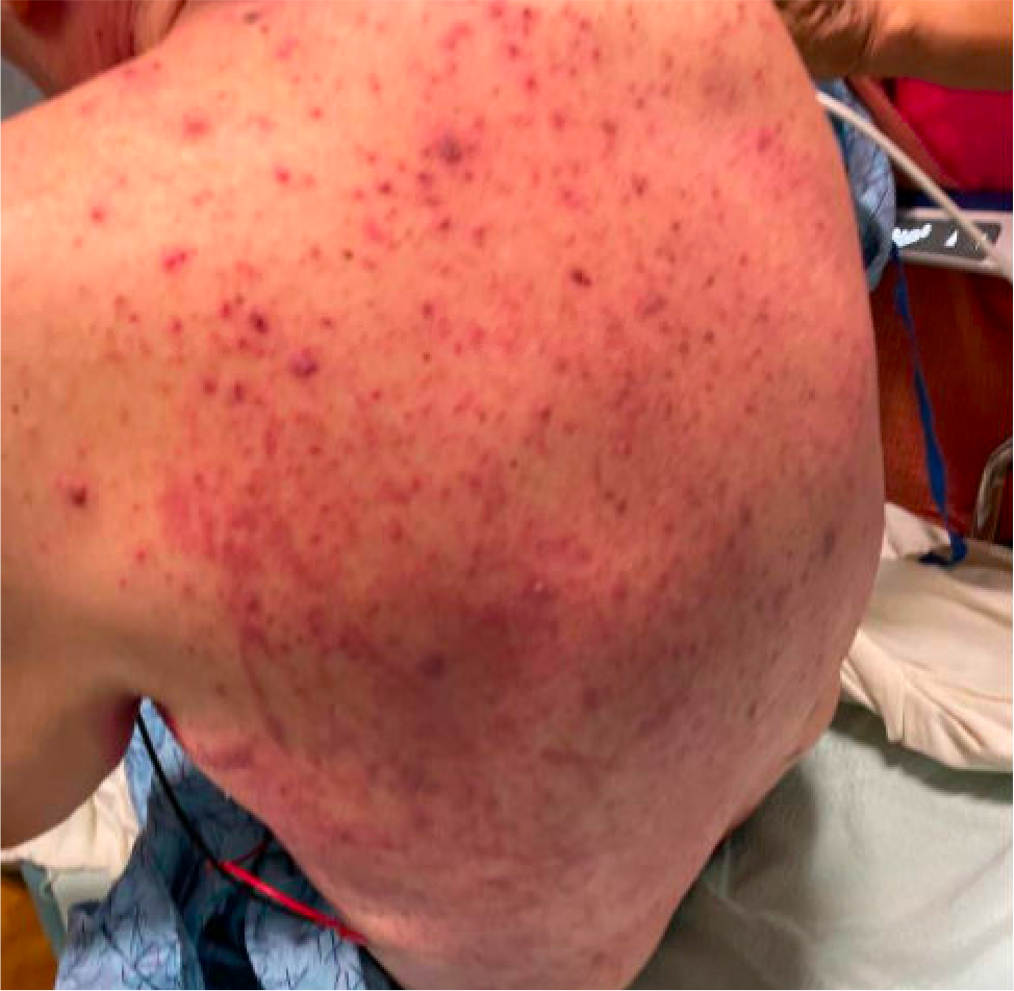
Case Presentation: A 80 year-old man with past medical history of frontotemporal dementia and seronegative rheumatoid arthritis (RA) presented with a maculopapular rash that started two weeks ago on his back that progressed to his chest, abdomen, neck, face, scalp, and arms. Physical exam also showed oral mucositis. For his RA, he had been on Methotrexate (MTX) subcutaneous 20mg weekly injections, but due to a shortage, one week prior to the start of the rash he was switched to oral MTX 20mg weekly. He was found to have pancytopenia with an absolute neutrophil count (ANC) of 800, and fevers (Temperature: 38.3C). He was started on IV Vancomycin and Cefepime, along with IV Acyclovir and oral Fluconazole for possible superinfection of HSV and Candidiasis. MTX level was normal. Infectious workup and HSV swab were negative, so all IV antimicrobials were discontinued without any further fevers. His ANC improved with Filgrastim and bacterial swab cultures eventually grew Serratia and Klebsiella. Dermatology recommended a two-week course with Cefpodoxime for concurrent gram-negative folliculitis. Ultimately, the patient’s rash improved and he was discharged with oral Fluconazole and Cefpodoxime. His MTX continued to be held until further follow up with rheumatology.
Discussion: Methotrexate’s antiproliferative activity has long been used to treat a wide range of conditions, including oncologic, rheumatologic, and dermatologic. However, it has also been associated with a wide array of adverse effects affecting those same organ systems they are intended to treat. Although our patient had concomitant bacterial and candidal skin infections, it was determined that the primary cause of the majority of his rash was due to methotrexate toxicity, especially in the setting of other adverse effects like pancytopenia and mucositis. While the subcutaneous and oral dose of MTX were both 20mg weekly, studies have shown that there is a higher incidence of adverse effects when it is taken orally. Furthermore, he was also taking Lansoprazole, which decreases elimination of MTX, thereby increasing his risk for toxicity. Though gastrointestinal symptoms are the most common side effect, up to 15% of patients on MTX have been shown to develop a nonspecific morbilliform rash, and in severe cases bullae formation and toxic epidermal necrolysis. While our patient previously tolerated this oral dose years ago prior to transitioning to subcutaneous injections, MTX toxicity has been shown to be related to drug dose, duration of exposure, and idiosyncratic. It is important for clinicians to be aware of these adverse effects when prescribing and treating patients with MTX.
Conclusions: 1. Consider Methotrexate toxicity in any patient taking Methotrexate who develops a new dermatologic condition. 2. Recognize that medication alterations, even if just changes in route of administration, can lead to adverse effects.